Procyanidin B2 3,3'-di-O-gallateCAS# 79907-44-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 79907-44-1 | SDF | Download SDF |
| PubChem ID | 124016 | Appearance | Brown powder |
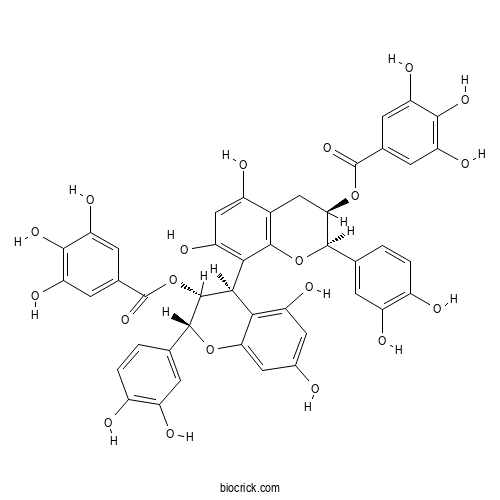
| Formula | C44H34O20 | M.Wt | 882.7 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Procyanidin B-2 3,3'-di-O-gallate | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(2R,3R)-2-(3,4-dihydroxyphenyl)-8-[(2R,3R,4R)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-(3,4,5-trihydroxybenzoyl)oxy-3,4-dihydro-2H-chromen-4-yl]-5,7-dihydroxy-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate | ||
| SMILES | C1C(C(OC2=C1C(=CC(=C2C3C(C(OC4=CC(=CC(=C34)O)O)C5=CC(=C(C=C5)O)O)OC(=O)C6=CC(=C(C(=C6)O)O)O)O)O)C7=CC(=C(C=C7)O)O)OC(=O)C8=CC(=C(C(=C8)O)O)O | ||
| Standard InChIKey | KTLUHRSHFRODPS-RIQPQZJCSA-N | ||
| Standard InChI | InChI=1S/C44H34O20/c45-19-11-26(51)34-32(12-19)61-40(16-2-4-22(47)25(50)6-16)42(64-44(60)18-9-30(55)38(58)31(56)10-18)36(34)35-27(52)14-23(48)20-13-33(62-43(59)17-7-28(53)37(57)29(54)8-17)39(63-41(20)35)15-1-3-21(46)24(49)5-15/h1-12,14,33,36,39-40,42,45-58H,13H2/t33-,36-,39-,40-,42-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Procyanidin B2 3,3'-di-O-gallate Dilution Calculator

Procyanidin B2 3,3'-di-O-gallate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1329 mL | 5.6644 mL | 11.3289 mL | 22.6578 mL | 28.3222 mL |
| 5 mM | 0.2266 mL | 1.1329 mL | 2.2658 mL | 4.5316 mL | 5.6644 mL |
| 10 mM | 0.1133 mL | 0.5664 mL | 1.1329 mL | 2.2658 mL | 2.8322 mL |
| 50 mM | 0.0227 mL | 0.1133 mL | 0.2266 mL | 0.4532 mL | 0.5664 mL |
| 100 mM | 0.0113 mL | 0.0566 mL | 0.1133 mL | 0.2266 mL | 0.2832 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6,7,4'-Trihydroxyflavanone
Catalog No.:BCN0706
CAS No.:189689-31-4
- Chrysocauloflavone I
Catalog No.:BCN0705
CAS No.:899789-51-6
- 3',3'''-Biapigenin
Catalog No.:BCN0704
CAS No.:151455-26-4
- Ochnaflavone
Catalog No.:BCN0703
CAS No.:50276-96-5
- Shanciol B
Catalog No.:BCN0702
CAS No.:208106-53-0
- 5,6-Dihydroxy-7,8-dimethoxyflavone
Catalog No.:BCN0701
CAS No.:76844-65-0
- Delicaflavone
Catalog No.:BCN0700
CAS No.:343569-15-3
- Luteolin 5-methyl ether
Catalog No.:BCN0699
CAS No.:58115-29-0
- Violanone
Catalog No.:BCN0698
CAS No.:52250-38-1
- Luteolin 5,3'-dimethyl ether
Catalog No.:BCN0697
CAS No.:62346-14-9
- (2S)-Pinocembrin 7-O-[2''-O-(5'''-O-trans-cinnamoyl)-β-D-apiofuranosyl]-β-D-glucoside
Catalog No.:BCN0696
CAS No.:773899-29-9
- Kayaflavone
Catalog No.:BCN0695
CAS No.:481-45-8
- 2'',3''-Dihydro-3',3'''-biapigenin
Catalog No.:BCN0708
CAS No.:151455-25-3
- 3',3'''-Binaringenin
Catalog No.:BCN0709
CAS No.:145399-99-1
- 2'',3''-Dihydroochnaflavone
Catalog No.:BCN0710
CAS No.:340997-02-6
- Procyanidin B5
Catalog No.:BCN0711
CAS No.:12798-57-1
- Procyanidin B2 3''-O-gallate
Catalog No.:BCN0712
CAS No.:73086-04-1
- 2"-O-Glucosylrutin
Catalog No.:BCN0713
CAS No.:55696-55-4
- 2,3-Dihydroamentoflavone
Catalog No.:BCN0714
CAS No.:34340-51-7
- Procyanidin B-5 3,3'-di-O-gallate
Catalog No.:BCN0715
CAS No.:106533-60-2
- Quercetin dimer
Catalog No.:BCN0716
CAS No.:167276-19-9
- 3'',4''-Di-O-p-coumaroylquercitrin
Catalog No.:BCN0717
CAS No.:437615-43-5
- Pinobanksin 3-(2-methyl)butyrate
Catalog No.:BCN0718
CAS No.:1221923-43-8
- Derrisisoflavone J
Catalog No.:BCN0719
CAS No.:2172624-67-6
Reynoutria Rhizomes as a Natural Source of SARS-CoV-2 Mpro Inhibitors-Molecular Docking and In Vitro Study.[Pubmed:34451839]
Pharmaceuticals (Basel). 2021 Jul 29;14(8). pii: ph14080742.
More than a year has passed since the world began to fight the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) responsible for the Coronavirus disease 2019 (COVID-19) pandemic, and still it spreads around the world, mutating at the same time. One of the sources of compounds with potential antiviral activity is Traditional Chinese Medicinal (TCM) plants used in China in the supportive treatment of COVID-19. Reynoutria japonica is important part of the Shu Feng Jie Du Granule/Capsule-TCM herbal formula, recommended by China Food and Drug Administration (CFDA) for treatment of patients with H1N1- and H5N9-induced acute lung injury and is also used in China to treat COVID-19, mainly combined with other remedies. In our study, 25 compounds from rhizomes of R. japonica and Reynoutria sachalinensis (related species), were docked into the binding site of SARS-CoV-2 main protease. Next, 11 of them (vanicoside A, vanicoside B, resveratrol, piceid, emodin, epicatechin, epicatechin gallate, epigallocatechin gallate, procyanidin B2, procyanidin C1, procyanidin B2 3,3'-di-O-gallate) as well as extracts and fractions from rhizomes of R. japonica and R. sachalinensis were tested in vitro using a fluorescent peptide substrate. Among the tested phytochemicals the best results were achieved for vanicoside A and vanicoside B with moderate inhibition of SARS-CoV-2 Mpro, IC50 = 23.10 microM and 43.59 microM, respectively. The butanol fractions of plants showed the strongest inhibition of SARS-CoV-2 Mpro (IC50 = 4.031 microg/mL for R. sachalinensis and IC50 = 7.877 microg/mL for R. japonica). As the main constituents of butanol fractions, besides the phenylpropanoid disaccharide esters (e.g., vanicosides), are highly polymerized procyanidins, we suppose that they could be responsible for their strong inhibitory properties. As inhibition of SARS-CoV-2 main protease could prevent the replication of the virus our research provides data that may explain the beneficial effects of R. japonica on COVID-19 and identify the most active compounds worthy of more extensive research.
Galloyl Group in B-type Proanthocyanidin Dimers Was Responsible for Its Differential Inhibitory Activity on 3T3-L1 Preadipocytes due to the Strong Lipid Raft-Perturbing Potency.[Pubmed:33891410]
J Agric Food Chem. 2021 May 5;69(17):5216-5225.
The effects of three B-type proanthocyanidin (PA) dimers covering procyanidin B2 (B-0g), procyanidin B2 3'-O-gallate (B-1g), and procyanidin B2 3,3'-di-O-gallate (B-2g) on 3T3-L1 preadipocyte differentiation and the underlying mechanisms were investigated. The results showed that digalloylated B-type PA dimers (B-2g) strongly inhibited 3T3-L1 preadipocyte differentiation through disrupting the integrity of the lipid raft structure and inhibiting the expression of peroxisome proliferator-activated receptor gamma (PPARgamma) and CCAAT/enhancer-binding protein alpha (C/EBPalpha) and then downregulating the expression of acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) factors, followed by B-1g, while B-0g had little effect. The different inhibitory effects were mainly due to the difference in the B-type PA dimer structure and the ability to interfere with lipid rafts. The greater the galloylation degree of B-type PA dimers, the stronger the ability to disrupt the lipid raft structure and oppose 3T3-L1 preadipocyte differentiation. In addition, galloylated B-type PA dimers had greater molecular hydrophobicity and topological polarity surface area and could penetrate into the lipid rafts to form multiple hydrogen bonds with the rafts by molecular dynamics simulation. These findings highlighted that the strong lipid raft-perturbing potency of galloylated B-type PA dimers was responsible for inhibition of 3T3-L1 preadipocyte differentiation.
Inhibitory effect of strawberry geranium (Saxifraga stolonifera) on Toll-like receptor 2-mediated inflammatory response in human skin keratinocytes.[Pubmed:33819504]
J Ethnopharmacol. 2021 Jul 15;275:114039.
ETHNOPHARMACOLOGICAL RELEVANCE: Strawberry geranium (Saxifraga stolonifera [L.] Meeb) has traditionally been used as a drug to treat skin disorders in Japan. However, little is known about its physiological effects on skin keratinocytes. AIM OF THE STUDY: We investigated the anti-inflammatory effects of a strawberry geranium extract (SGE) on human skin keratinocytes. MATERIALS AND METHODS: The human keratinocyte cell line, HaCaT, was treated with SGE, and then stimulated with tumor necrosis factor (TNF)-alpha. The expression of 207 genes related to the innate immune system was analyzed using DNA microarrays. The effect of SGE on the target proteins in primary human epidermal keratinocytes was confirmed by quantitative reverse transcription polymerase chain reaction and enzyme-linked immunosorbent assay. The mechanisms of action and active components involved in the suppressive effect of SGE were evaluated by fractionation and a transcription assay. RESULTS: The microarray analysis revealed that SGE primarily suppressed Toll-like receptor (TLR)2 expression through procyanidin B2 3,3'-di-O-gallate, without TLR2 downregulation, in TNF-alpha-stimulated HaCaT cells. SGE suppressed TLR2 expression and interleukin (IL)-8 production induced by TLR2 ligands in primary human epidermal keratinocytes and HaCaT cells. Multiple components downregulating TLR2 expression suppressed the Sp1 activity. CONCLUSIONS: We identified a novel physiological function of SGE, which suppresses TLR2 expression and TLR2-mediated inflammation in human skin keratinocytes. This study provides significant insights into the anti-inflammatory effect of SGE in human skin.


