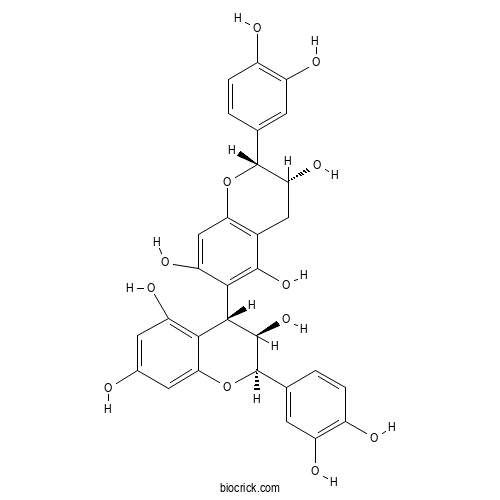
Procyanidin B5CAS# 12798-57-1 |
- Procyanidin B6
Catalog No.:BCN0895
CAS No.:12798-58-2
- Procyanidin B7
Catalog No.:BCN0896
CAS No.:12798-59-3
- Procyanidin B8
Catalog No.:BCN0897
CAS No.:12798-60-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 12798-57-1 | SDF | Download SDF |
| PubChem ID | 124017 | Appearance | Brown powder |
| Formula | C30H26O12 | M.Wt | 578.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Procyanidin B-5; Epicatechin-(4β→6)-epicatechin | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3R)-2-(3,4-dihydroxyphenyl)-6-[(2R,3R,4S)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-4-yl]-3,4-dihydro-2H-chromene-3,5,7-triol | ||
| SMILES | C1C(C(OC2=C1C(=C(C(=C2)O)C3C(C(OC4=CC(=CC(=C34)O)O)C5=CC(=C(C=C5)O)O)O)O)C6=CC(=C(C=C6)O)O)O | ||
| Standard InChIKey | GMISZFQPFDAPGI-CVJZBMGUSA-N | ||
| Standard InChI | InChI=1S/C30H26O12/c31-13-7-19(36)24-23(8-13)42-30(12-2-4-16(33)18(35)6-12)28(40)26(24)25-20(37)10-22-14(27(25)39)9-21(38)29(41-22)11-1-3-15(32)17(34)5-11/h1-8,10,21,26,28-40H,9H2/t21-,26-,28-,29-,30-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Procyanidin B5 Dilution Calculator

Procyanidin B5 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7286 mL | 8.643 mL | 17.2861 mL | 34.5722 mL | 43.2152 mL |
| 5 mM | 0.3457 mL | 1.7286 mL | 3.4572 mL | 6.9144 mL | 8.643 mL |
| 10 mM | 0.1729 mL | 0.8643 mL | 1.7286 mL | 3.4572 mL | 4.3215 mL |
| 50 mM | 0.0346 mL | 0.1729 mL | 0.3457 mL | 0.6914 mL | 0.8643 mL |
| 100 mM | 0.0173 mL | 0.0864 mL | 0.1729 mL | 0.3457 mL | 0.4322 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2'',3''-Dihydroochnaflavone
Catalog No.:BCN0710
CAS No.:340997-02-6
- 3',3'''-Binaringenin
Catalog No.:BCN0709
CAS No.:145399-99-1
- 2'',3''-Dihydro-3',3'''-biapigenin
Catalog No.:BCN0708
CAS No.:151455-25-3
- Procyanidin B2 3,3'-di-O-gallate
Catalog No.:BCN0707
CAS No.:79907-44-1
- 6,7,4'-Trihydroxyflavanone
Catalog No.:BCN0706
CAS No.:189689-31-4
- Chrysocauloflavone I
Catalog No.:BCN0705
CAS No.:899789-51-6
- 3',3'''-Biapigenin
Catalog No.:BCN0704
CAS No.:151455-26-4
- Ochnaflavone
Catalog No.:BCN0703
CAS No.:50276-96-5
- Shanciol B
Catalog No.:BCN0702
CAS No.:208106-53-0
- 5,6-Dihydroxy-7,8-dimethoxyflavone
Catalog No.:BCN0701
CAS No.:76844-65-0
- Delicaflavone
Catalog No.:BCN0700
CAS No.:343569-15-3
- Luteolin 5-methyl ether
Catalog No.:BCN0699
CAS No.:58115-29-0
- Procyanidin B2 3''-O-gallate
Catalog No.:BCN0712
CAS No.:73086-04-1
- 2"-O-Glucosylrutin
Catalog No.:BCN0713
CAS No.:55696-55-4
- 2,3-Dihydroamentoflavone
Catalog No.:BCN0714
CAS No.:34340-51-7
- Procyanidin B-5 3,3'-di-O-gallate
Catalog No.:BCN0715
CAS No.:106533-60-2
- Quercetin dimer
Catalog No.:BCN0716
CAS No.:167276-19-9
- 3'',4''-Di-O-p-coumaroylquercitrin
Catalog No.:BCN0717
CAS No.:437615-43-5
- Pinobanksin 3-(2-methyl)butyrate
Catalog No.:BCN0718
CAS No.:1221923-43-8
- Derrisisoflavone J
Catalog No.:BCN0719
CAS No.:2172624-67-6
- Epimedonin L
Catalog No.:BCN0720
CAS No.:2215102-38-6
- Derrisisoflavone H
Catalog No.:BCN0721
CAS No.:2172624-65-4
- Derrisisoflavone K
Catalog No.:BCN0722
CAS No.:2172624-68-7
- Derrisisoflavone I
Catalog No.:BCN0723
CAS No.:2172624-66-5
[Condensed tannins from roots of Indigofera stachyodes].[Pubmed:34467724]
Zhongguo Zhong Yao Za Zhi. 2021 Aug;46(16):4131-4138.
Eleven condensed tannins were isolated from the roots of Indigofera stachyodes by various column chromatography techniques including silica gel, octadecyl silica(ODS), Sephadex LH-20, and semi-preparative high performance liquid chromatography(HPLC). These compounds were identified on the basis of physicochemical properties, nuclear magnetic resonance(NMR) and mass spectrometry(MS) data as stachyotannin A(1), epicatechin-(2beta-->O-->7,4beta-->8)-epiafzelechin-(4beta-->8)-catechin(2), cinnamtannin D1(3), cinnamtannin B1(4), epicatechin-(2beta-->O-->7,4beta-->8)-epiafzelechin-(4alpha-->8)-epicatechin(5), gambiriin C(6), proanthocyanidin A1(7), proanthocyanidin A2(8), aesculitannin B(9), proanthocyanidin A4(10), and Procyanidin B5(11). Compound 1 is a new compound. Compounds 2-11 were isolated from Indigofera for the first time. Furthermore, compounds 1, 2, and 4-11 showed inhibitory effects on thrombin-induced ATP release in platelets.
Phytochemical investigation of Rumex thyrsiflorus Fingerh.[Pubmed:28605979]
Acta Biol Hung. 2017 Jun;68(2):232-236.
In the course of our pharmacological screening of Polygonaceae species occurring in the Carpathian Basin the extracts prepared from the roots of Rumex thyrsiflorus showed promising antiproliferative, xanthine oxidase inhibitory and antibacterial activities. The present work deals with the isolation of compounds from the root of the plant. After multistep separation process, four compounds were obtained from the n-hexane, chloroform and ethyl acetate soluble fractions of the methanol extract of the root. The structures of the isolated compounds were determined as 1-palmitoylglycerol, beta-sitosterol, (-)-epicatechin, and Procyanidin B5.
ISOLATION AND CHARACTERIZATION OF CHEMICAL CONSTITUENTS FROM CHRYSOPHYLLUM ALBIDUM G. DON-HOLL. STEM-BARK EXTRACTS AND THEIR ANTIOXIDANT AND ANTIBACTERIAL PROPERTIES.[Pubmed:28487910]
Afr J Tradit Complement Altern Med. 2016 Aug 12;13(5):182-189.
BACKGROUND: The plant, Chrysophyllum albidum is indigenous to Nigeria and its stem-bark has wide application in traditional medicine for the treatment of infections and oxidative stress related diseases. The aim of the study was to isolate the chemical constituents responsible for the antioxidant and antibacterial activity from the stem-bark of the plant in order to justify some of its ethnomedicinal uses. MATERIALS AND METHODS: Crude extract of stem-bark of Chrysophyllum albidum obtained from 80% ethanol was successively partitioned with ethyl acetate (EtOAc) and n-butanol. The solvent fractions and isolated compounds were tested for antioxidant property using 2-2-diphenyl-1-picrylhydrazyl. Antibacterial activities were also assessed by means of agar-diffusion and broth micro dilution methods. EtOAc fraction was repeatedly fractionated on column chromatography to afford four compounds and their chemical structures were established using NMR (1D and 2D) and MS. RESULTS: Chromatographic fractionation of EtOAc fraction with the highest antioxidant and antimicrobial activities afforded stigmasterol (1),: epicatechin (2),: epigallocatechin (3): and Procyanidin B5 (4).: Procyanidin B5 isolated for the first time from genus Chrysophyllum demonstrated the highest antioxidant activity with IC50 values of 8.8 muM and 11.20 muM in DPPH and nitric oxide assays respectively and equally demonstrated the highest inhibitory activity against Escherichia coli (MIC 156.25 mug/mL), Staphylococcus aureus (MIC 156.25 mug/mL), Pseudomonas aeruginosa (MIC 625 mug/mL) and Bacillus subtilis (MIC 156.25 mug/mL). CONCLUSION: The antibacterial and antioxidant activities of epicatechin, epigallocatechin and Procyanidin B5 isolated from Chrysophyllum albidum stem-bark validate the folkloric uses.
Diversity of cacao trees in Waslala, Nicaragua: associations between genotype spectra, product quality and yield potential.[Pubmed:23349790]
PLoS One. 2013;8(1):e54079.
The sensory quality and the contents of quality-determining chemical compounds in unfermented and fermented cocoa from 100 cacao trees (individual genotypes) representing groups of nine genotype spectra (GG), grown at smallholder plantings in the municipality of Waslala, Nicaragua, were evaluated for two successive harvest periods. Cocoa samples were fermented using a technique mimicking recommended on-farm practices. The sensory cocoa quality was assessed by experienced tasters, and seven major chemical taste compounds were quantified by near infrared spectrometry (NIRS). The association of the nine, partially admixed, genotype spectra with the analytical and sensory quality parameters was tested. The individual parameters were analyzed as a function of the factors GG and harvest (including the date of fermentation), individual trees within a single GG were used as replications. In fermented cocoa, significant GG-specific differences were observed for methylxanthines, theobromine-to-caffeine (T/C) ratio, total fat, Procyanidin B5 and epicatechin, as well as the sensory attributes global score, astringency, and dry fruit aroma, but differences related to harvest were also apparent. The potential cocoa yield was also highly determined by the individual GG, although there was significant tree-to-tree variation within every single GG. Non-fermented samples showed large harvest-to-harvest variation of their chemical composition, while differences between GG were insignificant. These results suggest that selection by the genetic background, represented here by groups of partially admixed genotype spectra, would be a useful strategy toward enhancing quality and yield of cocoa in Nicaragua. Selection by the GG within the local, genetically segregating populations of seed-propagated cacao, followed by clonal propagation of best-performing individuals of the selected GG could be a viable alternative to traditional propagation of cacao by seed from open pollination. Fast and gentle air-drying of the fermented beans and their permanent dry storage were an efficient and comparatively easy precondition for high cocoa quality.
In vitro inhibition of cytochrome P450 3A4 by Aronia melanocarpa constituents.[Pubmed:23250807]
Planta Med. 2013 Jan;79(2):137-41.
Extracts, subfractions, isolated anthocyanins and procyanidins, and two phenolic acids from aronia [Aronia melanocarpa] were investigated for their CYP3A4 inhibitory effects, using midazolam as the probe substrate and recombinant insect cell microsomes expressing CYP3A4 as the enzyme source. Procyanidin B5 was a considerably stronger CYP3A4 inhibitor in vitro than the isomeric procyanidin B2 and comparable to bergamottin, a known CYP3A4 inhibitor from grapefruit juice. The inhibitory activity of proanthocyanidin-containing fractions was correlated to the degree of polymerization. Among the anthocyanins, cyanidin 3-arabinoside showed stronger CYP3A4 inhibition than cyanidin 3-galactoside and cyanidin 3-glucoside. Thus, the ability to inhibit CYP3A4 in vitro seems to be influenced by the sugar unit linked to the anthocyanidin.
Polyphenolic profile and biological activity of Chinese hawthorn (Crataegus pinnatifida BUNGE) fruits.[Pubmed:23222867]
Molecules. 2012 Dec 6;17(12):14490-509.
Chinese hawthorn (Crataegus pinnatifida Bge.) fruits are rich in polyphenols (e.g., epicatechin, procyanidin B2, Procyanidin B5, procyanidin C1, hyperoside, isoquercitrin and chlorogenic acid)--active compounds that exert beneficial effects. This review summarizes all information available on polyphenolic content and methods for their quantification in Chinese hawthorn berries and the relationships between individual polyphenolic compounds as well. The influence of species or cultivars, the locality of cultivation, the stage of maturity, and extract preparation conditions on the polyphenolic content were discussed as well. Currently, only fruits of C. pinnatifida and C. pinnatifida var. major are included in the Chinese Pharmacopoeia. Recent trials have demonstrated the efficacy of Chinese hawthorn fruit in lowering blood cholesterol and the risk of cardiovascular diseases. The fruit has also demonstrated anti-inflammatory and anti-tumour activities. This review deals mainly with the biological activity of the fruit related to its antioxidant properties.
Constituents of Carpobrotus edulis inhibit P-glycoprotein of MDR1-transfected mouse lymphoma cells.[Pubmed:20393003]
Anticancer Res. 2010 Mar;30(3):829-35.
A bioassay-guided separation protocol, including the testing of the extracts, fractions and pure compounds for their ability to inhibit P-glycoprotein (the efflux pump responsible for the multidrug resistance of the used cell line) of mouse lymphoma cells containing the human efflux pump gene MDR1, led to the isolation of seven compounds from the chloroform and ethyl acetate soluble fractions of the methanolic extract of Carpobrotus edulis. The compounds were identified by 1D, 2D NMR and MS investigations as triterpens (beta-amyrin, uvaol and oleanolic acid), monogalactosyldiacylglycerol, catechin, epicatechin and Procyanidin B5. Uvaol was the most effective and promising compound in the reversal of multidrug resistance in MDR mouse lymphoma cell line.
Dietary polyphenols generate nitric oxide from nitrite in the stomach and induce smooth muscle relaxation.[Pubmed:19778575]
Toxicology. 2009 Nov 9;265(1-2):41-8.
Nitrite, considered a biological waste and toxic product, is being regarded as an important physiological molecule in nitric oxide (NO) biochemistry. Because the interaction of dietary phenolic compounds and nitrite would be kinetically (due to the high concentrations achieved) and thermodynamically (on basis of the redox potentials) feasible in the stomach, we have studied the potential reduction of nitrite by polyphenols present in several dietary sources. By measuring the time courses of *NO production in simulated gastric juice (pH 2), the efficiency of the compounds studied is as follows: Epicatechin-3-O-gallate>quercetin>procyanidin B8 dimer>oleuropein>procyanidin B2 dimer>chlorogenic acid>epicatechin>catechin>Procyanidin B5 dimer. The initial rates of *NO production fall in a narrow range (ca. 1-5 microMs(-1)) but the distinct kinetics of the decay of *NO signals suggest that competition reactions for *NO are operative. The proof of concept that, in the presence of nitrite, phenol-containing dietary products induce a strong increase of *NO in the stomach was established in an in vivo experiment with healthy volunteers consuming lettuce, onions, apples, wine, tea, berries and cherries. Moreover, selected mixtures of oleuropein and catechin with low nitrite (1 microM) were shown to induce muscle relaxation of stomach strips in a structure-dependent way. Data presented here brings strong support to the concept that polyphenols consumed in a variety of dietary products, under gastric conditions, reduce nitrite to *NO that, in turn, may exert a biological impact as a local relaxant.
Red wine polyphenols for cancer prevention.[Pubmed:19325788]
Int J Mol Sci. 2008 May;9(5):842-53.
Conventional cancer therapies, the second leading cause of death worldwide, result in serious side effects and, at best, merely extend the patient's lifespan by a few years. Searching for effective prevention is of high priority in both basic and clinical sciences. In recent decades natural products have been considered to be an important source of cancer chemopreventive agents. Red wine polyphenols, which consisted of various powerful antioxidants such as flavonoids and stilbenes, have been implicated in cancer prevention and that promote human health without recognizable side effects. Since resveratrol, a major component of red wine polyphenols, has been studied and reviewed extensively for its chemopreventive activity to interfere with the multi-stage carcinogenesis, this review focuses on recent progress in studies on cancer chemopreventive activities of red wine polyphenol extracts and fractions as well as other red wine polyphenols, like Procyanidin B5 analogues and myricetin.
Procyanidins from Myrothamnus flabellifolia.[Pubmed:18932087]
Nat Prod Res. 2008;22(14):1237-48.
From the ethyl acetate soluble fraction of an acetone-water extract of the twig tips of Myrothamnus flabellifolia Welw. (Myrothamnaceae), a variety of flavan-3-ols (epicatechin, epigallocatechin and their 3-O-galloylated analogues) and procyanidins (DP
Quantification of the polyphenols and triterpene acids in chinese hawthorn fruit by high-performance liquid chromatography.[Pubmed:16787000]
J Agric Food Chem. 2006 Jun 28;54(13):4574-81.
The levels of seven polyphenols (epicatechin, procyanidin B2, Procyanidin B5, procyanidin C1, hyperoside, isoquercitrin, and chlorogenic acid) and two triterpene acids (oleanolic acid and ursolic acid) in the matured fruits of Chinese hawthorn (Crataegus pinnatifida Bge. var. major N.E.Br.) were determined by high-performance liquid chromatography methods. The average contents of those constituents in 37 representative cultivars were 1405, 1505, 339, 684, 56, 41, 234, 952, and 147 microg/g fresh weight (FW), respectively. A significant inverse correlation between the procyanidin contents and the latitude of the geographical origin of the cultivars was observed (r = 0.3851, P < 0.02). Correlation analysis of the levels of the nine compounds in the 37 cultivars yielded a strong correlation (P < 0.001) between the individual levels of the four procyanidins and the sum of the procyanidins level (r = 0.7413-0.9898) and between the flavonoids and the chlorogenic acid (r = 0.5383-0.9212). The changes in level of the nine compounds in the hawthorn fruit were evaluated during maturation using the Hebei Dajinxing cultivar. Sixty-one days after blossom, the polyphenol level reached the highest point and the sum of the contents was 1.36 g/100 g FW.
HPLC coupled on-line to ESI-MS and a DPPH-based assay for the rapid identification of anti-oxidants in Butea superba.[Pubmed:16315486]
Phytochem Anal. 2005 Nov-Dec;16(6):422-8.
A reversed-phase HPLC coupled on-line to a radical scavenging detection system and MS/MS was developed in order to combine separation, activity determination and structural identification of anti-oxidants in complex mixtures in one run. The sample was separated by HPLC and the eluate split into two flows. The major portion was fed into an electrospray ionisation MS/MS system, while the minor part was mixed with a free radical, 2,2'-diphenyl-1-picrylhydrazyl (DPPH), and the reaction determined spectrophotometrically. The negative peaks, which indicated the presence of anti-oxidant activity, were monitored by measuring the decrease in absorbance at 517 nm. The developed method was successfully applied to the identification of anti-oxidant compounds in a fraction, obtained by solid-phase extraction, of an extract of a Thai medicinal plant, Butea superba Roxb. The anti-oxidant compounds were separated and identified as procyanidin B2, (-)-epicatechin and Procyanidin B5.
Antioxidant activity of polyphenols from seeds of Vitis amurensis in vitro.[Pubmed:11360672]
Acta Pharmacol Sin. 2000 Jul;21(7):633-6.
AIM: To study the antioxidant action of five polyphenols (+) catechin, procyanidin B2, Procyanidin B5, Procyanidin B5 3'-O-gallate, and amurensisin isolated from the seeds of Vitis amurensis. METHODS: The mouse liver homogenate lipid peroxidation assay was applied for the evaluation of the antioxidant activity in vitro. RESULTS: (+) Catechin, procyanidin B2, Procyanidin B5, Procyanidin B5 3'-O-gallate, and amurensisin showed antioxidant activity with the IC50 values of 0.47, 0.25, 0.10, 0.02, and 0.03 mmol/L, respectively. The IC50 value of vitamin E used as a positive control was 0.13 mmol/L. The structural activity relationship was also analyzed. Procyanidins carrying a galloyl group possessed higher anti-lipid peroxidation activities. All dimers were found to be more potent than the non-galloylated momomer such as (+) catechin. However, the activity of the 4-->6 linked dimer seemed more preferable than 4-->8 linked dimer. CONCLUSION: Procyanidin B5, Procyanidin B5 3'-O-gallate, and amurensisin showed a more antioxidant activity than vitamin E did, and their activity is dependent on their substitution and polymerization patterns.
Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation-promotion protocol and identification of procyanidin B5-3'-gallate as the most effective antioxidant constituent.[Pubmed:10469619]
Carcinogenesis. 1999 Sep;20(9):1737-45.
Procyanidins present in grape seeds are known to exert anti-inflammatory, anti-arthritic and anti-allergic activities, prevent skin aging, scavenge oxygen free radicals and inhibit UV radiation-induced peroxidation activity. Since most of these events are associated with the tumor promotion stage of carcinogenesis, these studies suggest that grape seed polyphenols and the procyanidins present therein could be anticarcinogenic and/or anti-tumor-promoting agents. Therefore, we assessed the anti-tumor-promoting effect of a polyphenolic fraction isolated from grape seeds (GSP) employing the 7,12-dimethylbenz[a]anthracene (DMBA)-initiated and 12-O-tetradecanoylphorbol 13-acetate (TPA)-promoted SENCAR mouse skin two-stage carcinogenesis protocol as a model system. Following tumor initiation with DMBA, topical application of GSP at doses of 0.5 and 1.5 mg/mouse/application to the dorsal initiated mouse skin resulted in a highly significant inhibition of TPA tumor promotion. The observed anti-tumor-promoting effects of GSP were dose dependent and were evident in terms of a reduction in tumor incidence (35 and 60% inhibition), tumor multiplicity (61 and 83% inhibition) and tumor volume (67 and 87% inhibition) at both 0.5 and 1.5 mg GSP, respectively. Based on these results, we directed our efforts to separate and identify the individual polyphenols present in GSP and assess their antioxidant activity in terms of inhibition of epidermal lipid peroxidation. Employing HPLC followed by comparison with authentic standards for retention times in HPLC profiles, physiochemical properties and spectral analysis, nine individual polyphenols were identified as catechin, epicatechin, procyanidins B1-B5 and C1 and Procyanidin B5-3'-gallate. Five of these individual polyphenols with evident structural differences, namely catechin, procyanidin B2, Procyanidin B5, procyanidin C1 and Procyanidin B5-3'-gallate, were assessed for antioxidant activity. All of them significantly inhibited epidermal lipid peroxidation, albeit to different levels. A structure-activity relationship study showed that with an increase in the degree of polymerization in polyphenol structure, the inhibitory potential towards lipid peroxidation increased. In addition, the position of linkage between inter-flavan units also influences lipid peroxidation activity; procyanidin isomers with a 4-6 linkage showed stronger inhibitory activity than isomers with a 4-8 linkage. A sharp increase in the inhibition of epidermal lipid peroxidation was also evident when a gallate group was linked at the 3'-hydroxy position of a procyanidin dimer. Procyanidin B5-3'-gallate showed the most potent antioxidant activity with an IC(50) of 20 microM in an epidermal lipid peroxidation assay. Taken together, for the first time these results show that grape seed polyphenols possess high anti-tumor-promoting activity due to the strong antioxidant effect of procyanidins present therein. In summary, grape seed polyphenols in general, and Procyanidin B5-3'-gallate in particular, should be studied in more detail to be developed as cancer chemopreventive and/or anticarcinogenic agents.


