6alpha-HydroxymaackiainCAS# 61218-44-8 |
Quality Control & MSDS
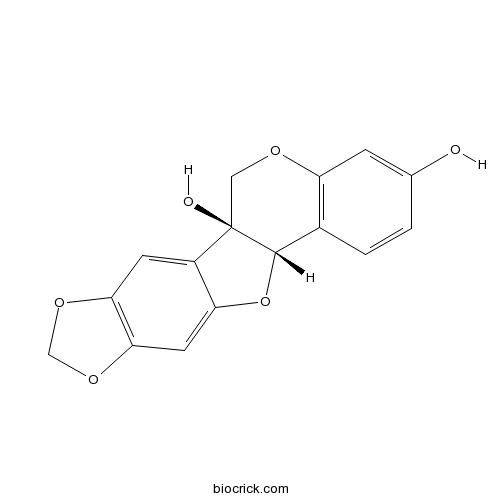
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 61218-44-8 | SDF | Download SDF |
| PubChem ID | 182285 | Appearance | Powder |
| Formula | C16H12O6 | M.Wt | 300.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | C1C2(C(C3=C(O1)C=C(C=C3)O)OC4=CC5=C(C=C42)OCO5)O | ||
| Standard InChIKey | GLMPLZUBQDAZEN-JKSUJKDBSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 6alpha-Hydroxymaackiain is a natural product from Derris robusta. |
| Structure Identification | Canadian Journal of Botany, 1981, 59(4): 547-548.Demethylation of the phytoalexin pisatin by Stemphylium botryosum[Reference: WebLink]The initial product formed from pisatin by Stemphylium botryosum was identified as 3,6a-dihydroxy-8,9-methylenedioxypterocarpan(6alpha-Hydroxymaackiain) by spectrophotometric, thin-layer chromatographic and gas–liquid chromatographic comparisons with an authentic standard. |

6alpha-Hydroxymaackiain Dilution Calculator

6alpha-Hydroxymaackiain Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.33 mL | 16.65 mL | 33.3 mL | 66.6001 mL | 83.2501 mL |
| 5 mM | 0.666 mL | 3.33 mL | 6.66 mL | 13.32 mL | 16.65 mL |
| 10 mM | 0.333 mL | 1.665 mL | 3.33 mL | 6.66 mL | 8.325 mL |
| 50 mM | 0.0666 mL | 0.333 mL | 0.666 mL | 1.332 mL | 1.665 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.333 mL | 0.666 mL | 0.8325 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Uzarigenin digitaloside
Catalog No.:BCN4613
CAS No.:61217-80-9
- Quinine HCl Dihydrate
Catalog No.:BCC4933
CAS No.:6119-47-7
- Grandifloroside
Catalog No.:BCN4133
CAS No.:61186-24-1
- 4-(3,4-Dihydroxyphenyl)-2-butanone
Catalog No.:BCN4132
CAS No.:61152-62-3
- 6alpha-Hydroxymedicarpin
Catalog No.:BCN3939
CAS No.:61135-92-0
- 3,9-Dihydroxypterocarpan
Catalog No.:BCN4131
CAS No.:61135-91-9
- Epipterosin L 2'-O-glucoside
Catalog No.:BCN4614
CAS No.:61117-89-3
- Bromhexine hydrochloride
Catalog No.:BCC8898
CAS No.:611-75-6
- (R)-Mandelic acid
Catalog No.:BCN8532
CAS No.:611-71-2
- Tectoridin
Catalog No.:BCN1020
CAS No.:611-40-5
- 4-Phenyl-1,2,3,4-tetrahydroisoquinoline hydrochloride
Catalog No.:BCC6769
CAS No.:6109-35-9
- Icotinib
Catalog No.:BCC4473
CAS No.:610798-31-7
- 11-Hydroxybisabola-1,3,5-trien-9-one
Catalog No.:BCN7530
CAS No.:61235-23-2
- Denudadione C
Catalog No.:BCN6608
CAS No.:61240-34-4
- AZD1080
Catalog No.:BCC4508
CAS No.:612487-72-6
- PTP1B-IN-1
Catalog No.:BCC5506
CAS No.:612530-44-6
- Cannabispiran
Catalog No.:BCN4134
CAS No.:61262-81-5
- Vitexilactone
Catalog No.:BCN4135
CAS No.:61263-49-8
- Acteoside
Catalog No.:BCN4136
CAS No.:61276-17-3
- Boc-D-Phe(4-NO2)-OH
Catalog No.:BCC3276
CAS No.:61280-75-9
- Schizandrin B
Catalog No.:BCN1022
CAS No.:61281-37-6
- Schizandrin A
Catalog No.:BCN1021
CAS No.:61281-38-7
- AKT inhibitor VIII
Catalog No.:BCC1334
CAS No.:612847-09-3
- 2-(Phenylmethoxy)-naphthalene
Catalog No.:BCC8485
CAS No.:613-62-7
Affinity chromatography, substrate/product specificity, and amino acid sequence analysis of an isoflavone O-methyltransferase from alfalfa (Medicago sativa L.).[Pubmed:8951042]
Arch Biochem Biophys. 1996 Dec 1;336(1):121-9.
Isoflavone O-methyltransferase (IOMT) is a key enzyme in the biosynthesis of the phytoalexin medicarpin in alfalfa. In vivo, the B-ring 4'-hydroxyl group of the isoflavone daidzein is methylated. Surprisingly, the O-methyltransferase activity measured in vitro preferentially methylates the A-ring 7-hydroxyl group, a reaction that probably does not occur in vivo. To resolve this anomaly, we are attempting to clone the alfalfa IOMT. A substrate-based affinity chromatographic system was developed to purify the enzyme (molecular weight 41 kDa) to near homogeneity. Four internal peptide sequences were obtained from the purified protein, one of which has high (72%) sequence identity to a region of a catechol O-methyltransferase from barley. All four internal peptides, respectively, have about 55% amino acid sequence identity to four regions of 6alpha-Hydroxymaackiain 3-O-methyltransferase from Pisum sativum, but have no sequence identity to alfalfa caffeic acid 3-O-methyltransferase or chalcone 2'-O-methyltransferase. The purified IOMT has substrate specificity toward isoflavones with a free 7-hydroxyl group, but can also methylate the 5-hydroxyl group of genistein.


