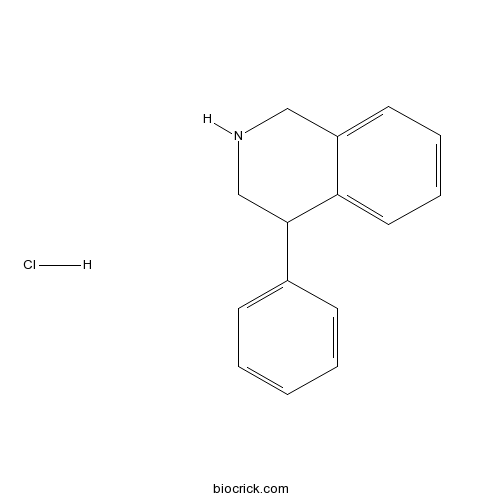
4-Phenyl-1,2,3,4-tetrahydroisoquinoline hydrochlorideDA release inhibitor CAS# 6109-35-9 |
- Verteporfin
Catalog No.:BCC3690
CAS No.:129497-78-5
- Methylcobalamin
Catalog No.:BCC5188
CAS No.:13422-55-4
- Miglustat hydrochloride
Catalog No.:BCC5186
CAS No.:210110-90-0
- Amifampridine
Catalog No.:BCC5185
CAS No.:54-96-6
- Miglustat
Catalog No.:BCC5187
CAS No.:72599-27-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6109-35-9 | SDF | Download SDF |
| PubChem ID | 12999296 | Appearance | Powder |
| Formula | C15H16ClN | M.Wt | 245.75 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO and to 25 mM in water | ||
| Chemical Name | 4-phenyl-1,2,3,4-tetrahydroisoquinoline;hydrochloride | ||
| SMILES | C1C(C2=CC=CC=C2CN1)C3=CC=CC=C3.Cl | ||
| Standard InChIKey | YYCOJKPDBCMVPP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H15N.ClH/c1-2-6-12(7-3-1)15-11-16-10-13-8-4-5-9-14(13)15;/h1-9,15-16H,10-11H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of release of dopamine induced by methamphetamine. |

4-Phenyl-1,2,3,4-tetrahydroisoquinoline hydrochloride Dilution Calculator

4-Phenyl-1,2,3,4-tetrahydroisoquinoline hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0692 mL | 20.3459 mL | 40.6918 mL | 81.3835 mL | 101.7294 mL |
| 5 mM | 0.8138 mL | 4.0692 mL | 8.1384 mL | 16.2767 mL | 20.3459 mL |
| 10 mM | 0.4069 mL | 2.0346 mL | 4.0692 mL | 8.1384 mL | 10.1729 mL |
| 50 mM | 0.0814 mL | 0.4069 mL | 0.8138 mL | 1.6277 mL | 2.0346 mL |
| 100 mM | 0.0407 mL | 0.2035 mL | 0.4069 mL | 0.8138 mL | 1.0173 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Icotinib
Catalog No.:BCC4473
CAS No.:610798-31-7
- Isobonducellin
Catalog No.:BCN4130
CAS No.:610778-85-3
- Boc-His(Nτ-Me)-OH
Catalog No.:BCC2684
CAS No.:61070-22-2
- L-Thyroxine sodium salt pentahydrate
Catalog No.:BCC4283
CAS No.:6106-07-6
- N-Desmethylclozapine
Catalog No.:BCC6887
CAS No.:6104-71-8
- Teicoplanin
Catalog No.:BCC4731
CAS No.:61036-62-2
- c-JUN peptide
Catalog No.:BCC8085
CAS No.:610273-01-3
- Ferulamide
Catalog No.:BCN4129
CAS No.:61012-31-5
- Succinylcholine Chloride Dihydrate
Catalog No.:BCC4564
CAS No.:6101-15-1
- Isoacetovanillone
Catalog No.:BCN7166
CAS No.:6100-74-9
- Tenuazonic acid
Catalog No.:BCN1859
CAS No.:610-88-8
- H-Leu-OH
Catalog No.:BCC2968
CAS No.:61-90-5
- Tectoridin
Catalog No.:BCN1020
CAS No.:611-40-5
- (R)-Mandelic acid
Catalog No.:BCN8532
CAS No.:611-71-2
- Bromhexine hydrochloride
Catalog No.:BCC8898
CAS No.:611-75-6
- Epipterosin L 2'-O-glucoside
Catalog No.:BCN4614
CAS No.:61117-89-3
- 3,9-Dihydroxypterocarpan
Catalog No.:BCN4131
CAS No.:61135-91-9
- 6alpha-Hydroxymedicarpin
Catalog No.:BCN3939
CAS No.:61135-92-0
- 4-(3,4-Dihydroxyphenyl)-2-butanone
Catalog No.:BCN4132
CAS No.:61152-62-3
- Grandifloroside
Catalog No.:BCN4133
CAS No.:61186-24-1
- Quinine HCl Dihydrate
Catalog No.:BCC4933
CAS No.:6119-47-7
- Uzarigenin digitaloside
Catalog No.:BCN4613
CAS No.:61217-80-9
- 6alpha-Hydroxymaackiain
Catalog No.:BCN3947
CAS No.:61218-44-8
- 11-Hydroxybisabola-1,3,5-trien-9-one
Catalog No.:BCN7530
CAS No.:61235-23-2
Resolution and absolute stereochemistry of 6,7-dimethoxy-4-phenyl- 1,2,3,4-tetrahydroisoquinoline. The crystal structure of the R-hydrochloride salt form.[Pubmed:1599795]
Acta Chem Scand. 1992 Jan;46(1):54-9.
Stereospecific multistep synthesis and resolution of 6,7-dimethoxy-4- phenyl-1,2,3,4-tetrahydroisoquinoline (3) has been achieved from its racemic base. The absolute configurations of the optical antipodes converted into their hydrochloride salt forms have been determined by X-ray diffractometric analysis, thus permitting assignment of the antipodes as the (+)-(4R)-3 and (-)-(4S)-3 enantiomers. The crystal structures of the two enantiomers are related as mirror images and only the (4R)-3.HCl form has been fully determined by three-dimensional X-ray diffraction. In the solid state, the carbon atoms of the two methoxy groups deviate slightly from the benzene-ring plane and the chirally oriented phenyl substituent is almost perpendicularly tilted out of conjugation with the isoquinoline system. Examination of the enantiomers in several biochemical tests for 5-HT, NE and DA uptake inhibition-activity revealed an exclusive preference for the (4S)-enantiomer. These results are in accord with previous suggestions that the S-configurational state of the 4-phenyl substituent is important for biological activity.
4-Phenyltetrahydroisoquinoline, but not nomifensine or cocaine, inhibits methamphetamine-induced dopamine release.[Pubmed:8405121]
Eur J Pharmacol. 1993 Aug 10;240(1):51-6.
The inhibitory effect of 4-phenyltetrahydroisoquinoline (4-PTIQ) on methamphetamine-induced dopamine release in the rat nucleus accumbens was investigated using a brain microdialysis method. Methamphetamine (10(-6) M) infusion through a microdialysis probe induced the release of dopamine. Although the uptake inhibitors, cocaine (3 x 10(-6) M) and nomifensine (10(-6) M), failed to block dopamine release, 4-PTIQ (10(-6 M) inhibited the dopamine-releasing effect of methamphetamine. 4-PTIQ did not affect the elevation of the extracellular dopamine level induced by high concentrations of nomifensine (10(-5) M) and cocaine (3 x 10(-5) M). 4-PTIQ was the weakest inhibitor of [3H]dopamine uptake by rat striatal synaptosomes. These results suggest that 4-PTIQ is a selective antagonist against the dopamine-releasing effect of methamphetamine in the nucleus accumbens.


