H-Leu-OHCAS# 61-90-5 |
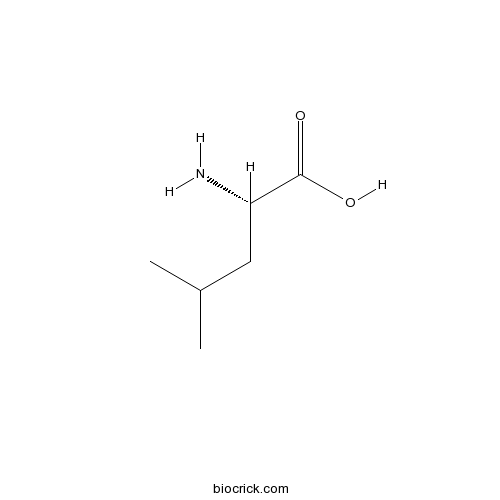
- H-D-Leu-OH
Catalog No.:BCC2975
CAS No.:328-38-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 61-90-5 | SDF | Download SDF |
| PubChem ID | 6106 | Appearance | Powder |
| Formula | C6H13NO2 | M.Wt | 131.2 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | L-leucine; Leucine; 61-90-5; (S)-Leucine; (S)-2-Amino-4-methylpentanoic acid; | ||
| Solubility | H2O : 8.33 mg/mL (63.51 mM; Need ultrasonic) | ||
| Chemical Name | (2S)-2-amino-4-methylpentanoic acid | ||
| SMILES | CC(C)CC(C(=O)O)N | ||
| Standard InChIKey | ROHFNLRQFUQHCH-YFKPBYRVSA-N | ||
| Standard InChI | InChI=1S/C6H13NO2/c1-4(2)3-5(7)6(8)9/h4-5H,3,7H2,1-2H3,(H,8,9)/t5-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | L-Leucine is an essential amino acid for the human body, L-Leucine improves the anemia and developmental defects associated with Diamond-Blackfan anemia and del(5q) MDS by activating the mTOR pathway, and an excess of dietary l-leucine has been shown to retard the growth of rats fed low-protein diets or diets deficient in isoleucine. |
| Targets | mTOR | p53 |
| In vitro | L-Leucine improves the anemia and developmental defects associated with Diamond-Blackfan anemia and del(5q) MDS by activating the mTOR pathway.[Pubmed: 22734070 ]Blood. 2012 Sep 13;120(11):2214-24.Haploinsufficiency of ribosomal proteins (RPs) has been proposed to be the common basis for the anemia observed in Diamond-Blackfan anemia (DBA) and myelodysplastic syndrome with loss of chromosome 5q [del(5q) MDS]. We have modeled DBA and del(5q) MDS in zebrafish using antisense morpholinos to rps19 and rps14, respectively, and have demonstrated that, as in humans, haploinsufficient levels of these proteins lead to a profound anemia. |
| In vivo | L-Leucine, an isoleucine antagonist in the rat[Reference: WebLink]Arch Biochem Biophys. 1955 Jul;57(1):1-12.
|
| Kinase Assay | Stimulation of mTORC1 with L-leucine rescues defects associated with Roberts syndrome[Pubmed: 24098154 ]PLoS Genet. 2013;9(10):e1003857.Roberts syndrome (RBS) is a human disease characterized by defects in limb and craniofacial development and growth and mental retardation. RBS is caused by mutations in ESCO2, a gene which encodes an acetyltransferase for the cohesin complex. While the essential role of the cohesin complex in chromosome segregation has been well characterized, it plays additional roles in DNA damage repair, chromosome condensation, and gene expression. The developmental phenotypes of Roberts syndrome and other cohesinopathies suggest that gene expression is impaired during embryogenesis. It was previously reported that ribosomal RNA production and protein translation were impaired in immortalized RBS cells. It was speculated that cohesin binding at the rDNA was important for nucleolar form and function. |
| Animal Research | Dietary L-leucine improves the anemia in a mouse model for Diamond-Blackfan anemia.[Pubmed: 22791294 ]Blood. 2012 Sep 13;120(11):2225-8.Diamond-Blackfan anemia (DBA) is a congenital erythroid hypoplasia caused by a functional haploinsufficiency of genes encoding for ribosomal proteins. Recently, a case study reported a patient who became transfusion-independent in response to treatment with the amino acid L-Leucine. |

H-Leu-OH Dilution Calculator

H-Leu-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.622 mL | 38.1098 mL | 76.2195 mL | 152.439 mL | 190.5488 mL |
| 5 mM | 1.5244 mL | 7.622 mL | 15.2439 mL | 30.4878 mL | 38.1098 mL |
| 10 mM | 0.7622 mL | 3.811 mL | 7.622 mL | 15.2439 mL | 19.0549 mL |
| 50 mM | 0.1524 mL | 0.7622 mL | 1.5244 mL | 3.0488 mL | 3.811 mL |
| 100 mM | 0.0762 mL | 0.3811 mL | 0.7622 mL | 1.5244 mL | 1.9055 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
H-Leu-OH
- Zoxazolamine
Catalog No.:BCC4751
CAS No.:61-80-3
- 4-Aminohippuric Acid
Catalog No.:BCC4753
CAS No.:61-78-9
- Phenylephrine HCl
Catalog No.:BCC4335
CAS No.:61-76-7
- Mefenamic Acid
Catalog No.:BCC4433
CAS No.:61-68-7
- Papaverine Hydrochloride
Catalog No.:BCC8348
CAS No.:61-25-6
- Adenosine 5'-monophosphate
Catalog No.:BCC8809
CAS No.:61-19-8
- Dibucaine (Cinchocaine) HCl
Catalog No.:BCC3760
CAS No.:61-12-1
- 2-Methoxycinnamic acid
Catalog No.:BCN5038
CAS No.:6099-03-2
- Geraniin
Catalog No.:BCN2402
CAS No.:60976-49-0
- Bz-Glu-OH
Catalog No.:BCC2922
CAS No.:6094-36-6
- YZ9
Catalog No.:BCC8001
CAS No.:6093-71-6
- 6-Hydroxycoumarin
Catalog No.:BCC9207
CAS No.:6093-68-1
- Tenuazonic acid
Catalog No.:BCN1859
CAS No.:610-88-8
- Isoacetovanillone
Catalog No.:BCN7166
CAS No.:6100-74-9
- Succinylcholine Chloride Dihydrate
Catalog No.:BCC4564
CAS No.:6101-15-1
- Ferulamide
Catalog No.:BCN4129
CAS No.:61012-31-5
- c-JUN peptide
Catalog No.:BCC8085
CAS No.:610273-01-3
- Teicoplanin
Catalog No.:BCC4731
CAS No.:61036-62-2
- N-Desmethylclozapine
Catalog No.:BCC6887
CAS No.:6104-71-8
- L-Thyroxine sodium salt pentahydrate
Catalog No.:BCC4283
CAS No.:6106-07-6
- Boc-His(Nτ-Me)-OH
Catalog No.:BCC2684
CAS No.:61070-22-2
- Isobonducellin
Catalog No.:BCN4130
CAS No.:610778-85-3
- Icotinib
Catalog No.:BCC4473
CAS No.:610798-31-7
- 4-Phenyl-1,2,3,4-tetrahydroisoquinoline hydrochloride
Catalog No.:BCC6769
CAS No.:6109-35-9
Stimulation of mTORC1 with L-leucine rescues defects associated with Roberts syndrome.[Pubmed:24098154]
PLoS Genet. 2013;9(10):e1003857.
Roberts syndrome (RBS) is a human disease characterized by defects in limb and craniofacial development and growth and mental retardation. RBS is caused by mutations in ESCO2, a gene which encodes an acetyltransferase for the cohesin complex. While the essential role of the cohesin complex in chromosome segregation has been well characterized, it plays additional roles in DNA damage repair, chromosome condensation, and gene expression. The developmental phenotypes of Roberts syndrome and other cohesinopathies suggest that gene expression is impaired during embryogenesis. It was previously reported that ribosomal RNA production and protein translation were impaired in immortalized RBS cells. It was speculated that cohesin binding at the rDNA was important for nucleolar form and function. We have explored the hypothesis that reduced ribosome function contributes to RBS in zebrafish models and human cells. Two key pathways that sense cellular stress are the p53 and mTOR pathways. We report that mTOR signaling is inhibited in human RBS cells based on the reduced phosphorylation of the downstream effectors S6K1, S6 and 4EBP1, and this correlates with p53 activation. Nucleoli, the sites of ribosome production, are highly fragmented in RBS cells. We tested the effect of inhibiting p53 or stimulating mTOR in RBS cells. The rescue provided by mTOR activation was more significant, with activation rescuing both cell division and cell death. To study this cohesinopathy in a whole animal model we used ESCO2-mutant and morphant zebrafish embryos, which have developmental defects mimicking RBS. Consistent with RBS patient cells, the ESCO2 mutant embryos show p53 activation and inhibition of the TOR pathway. Stimulation of the TOR pathway with L-leucine rescued many developmental defects of ESCO2-mutant embryos. Our data support the idea that RBS can be attributed in part to defects in ribosome biogenesis, and stimulation of the TOR pathway has therapeutic potential.
L-Leucine improves the anemia and developmental defects associated with Diamond-Blackfan anemia and del(5q) MDS by activating the mTOR pathway.[Pubmed:22734070]
Blood. 2012 Sep 13;120(11):2214-24.
Haploinsufficiency of ribosomal proteins (RPs) has been proposed to be the common basis for the anemia observed in Diamond-Blackfan anemia (DBA) and myelodysplastic syndrome with loss of chromosome 5q [del(5q) MDS]. We have modeled DBA and del(5q) MDS in zebrafish using antisense morpholinos to rps19 and rps14, respectively, and have demonstrated that, as in humans, haploinsufficient levels of these proteins lead to a profound anemia. To address the hypothesis that RP loss results in impaired mRNA translation, we treated Rps19 and Rps14-deficient embryos with the amino acid L-leucine, a known activator of mRNA translation. This resulted in a striking improvement of the anemia associated with RP loss. We confirmed our findings in primary human CD34(+) cells, after shRNA knockdown of RPS19 and RPS14. Furthermore, we showed that loss of Rps19 or Rps14 activates the mTOR pathway, and this is accentuated by L-leucine in both Rps19 and Rps14 morphants. This effect could be abrogated by rapamycin suggesting that mTOR signaling may be responsible for the improvement in anemia associated with L-leucine. Our studies support the rationale for ongoing clinical trials of L-leucine as a therapeutic agent for DBA, and potentially for patients with del(5q) MDS.
Dietary L-leucine improves the anemia in a mouse model for Diamond-Blackfan anemia.[Pubmed:22791294]
Blood. 2012 Sep 13;120(11):2225-8.
Diamond-Blackfan anemia (DBA) is a congenital erythroid hypoplasia caused by a functional haploinsufficiency of genes encoding for ribosomal proteins. Recently, a case study reported a patient who became transfusion-independent in response to treatment with the amino acid L-leucine. Therefore, we have validated the therapeutic effect of L-leucine using our recently generated mouse model for RPS19-deficient DBA. Administration of L-leucine significantly improved the anemia in Rps19-deficient mice (19% improvement in hemoglobin concentration; 18% increase in the number of erythrocytes), increased the bone marrow cellularity, and alleviated stress hematopoiesis. Furthermore, the therapeutic response to L-leucine appeared specific for Rps19-deficient hematopoiesis and was associated with down-regulation of p53 activity. Our study supports the rationale for clinical trials of L-leucine as a therapeutic agent for DBA.


