8-ShogaolCAS# 36700-45-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 36700-45-5 | SDF | Download SDF |
| PubChem ID | 6442560 | Appearance | Colorless - yellowish viscous liquid |
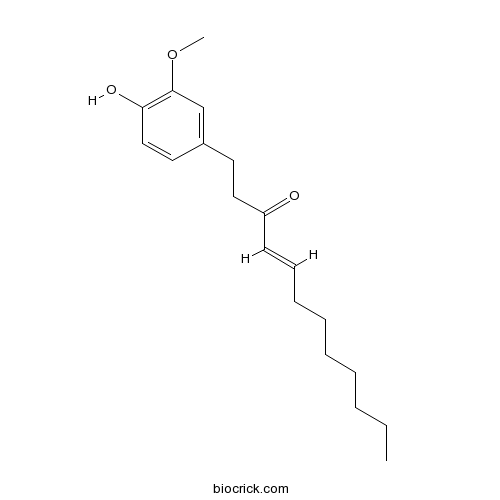
| Formula | C19H28O3 | M.Wt | 304.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in chloroform and methanol; insoluble in water | ||
| Chemical Name | (E)-1-(4-hydroxy-3-methoxyphenyl)dodec-4-en-3-one | ||
| SMILES | CCCCCCCC=CC(=O)CCC1=CC(=C(C=C1)O)OC | ||
| Standard InChIKey | LGZSMXJRMTYABD-MDZDMXLPSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 8-Shogaol can induce apoptosis in a time- and concentration-dependent manner by reactive oxygen species production and depletion of glutathione in HL-60 cells. |
| Targets | ROS |

8-Shogaol Dilution Calculator

8-Shogaol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2852 mL | 16.4258 mL | 32.8515 mL | 65.703 mL | 82.1288 mL |
| 5 mM | 0.657 mL | 3.2852 mL | 6.5703 mL | 13.1406 mL | 16.4258 mL |
| 10 mM | 0.3285 mL | 1.6426 mL | 3.2852 mL | 6.5703 mL | 8.2129 mL |
| 50 mM | 0.0657 mL | 0.3285 mL | 0.657 mL | 1.3141 mL | 1.6426 mL |
| 100 mM | 0.0329 mL | 0.1643 mL | 0.3285 mL | 0.657 mL | 0.8213 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Benzyl 2,4-dihydroxyphenyl ketone
Catalog No.:BCC8867
CAS No.:3669-41-8
- Isorhamnetin 3-glucuronide
Catalog No.:BCN2709
CAS No.:36687-76-0
- Pulchinenoside E
Catalog No.:BCN8165
CAS No.:366814-43-9
- Cussosaponin C
Catalog No.:BCN2895
CAS No.:366814-42-8
- Rivaroxaban
Catalog No.:BCC2292
CAS No.:366789-02-8
- 1-Hexadecanol
Catalog No.:BCC4616
CAS No.:36653-82-4
- Semialactone
Catalog No.:BCN5422
CAS No.:366450-46-6
- 1,4-Benzodioxan-2-carboxylic acid
Catalog No.:BCC8421
CAS No.:3663-80-7
- Mubritinib (TAK 165)
Catalog No.:BCC4513
CAS No.:366017-09-6
- AY 9944 dihydrochloride
Catalog No.:BCC3940
CAS No.:366-93-8
- Procarbazine HCl
Catalog No.:BCC4452
CAS No.:366-70-1
- H-Arg-OEt.2HCl
Catalog No.:BCC2860
CAS No.:36589-29-4
- Beta-Solamarine
Catalog No.:BCN2693
CAS No.:3671-38-3
- Boc-Phe-Osu
Catalog No.:BCC2601
CAS No.:3674-06-4
- Boc-D-Phe-Osu
Catalog No.:BCC2600
CAS No.:3674-18-8
- Lurasidone
Catalog No.:BCC9013
CAS No.:367514-87-2
- Lurasidone HCl
Catalog No.:BCC4458
CAS No.:367514-88-3
- AR-M 1896
Catalog No.:BCC5931
CAS No.:367518-31-8
- 10-Shogaol
Catalog No.:BCN3267
CAS No.:36752-54-2
- 4-Aminophthalimide
Catalog No.:BCC8689
CAS No.:3676-85-5
- MRS 2279
Catalog No.:BCC5880
CAS No.:367909-40-8
- Ribavirin
Catalog No.:BCC4935
CAS No.:36791-04-5
- Vitexin
Catalog No.:BCN5423
CAS No.:3681-93-4
- Puerarin
Catalog No.:BCN5958
CAS No.:3681-99-0
Induction of apoptosis by [8]-shogaol via reactive oxygen species generation, glutathione depletion, and caspase activation in human leukemia cells.[Pubmed:20163181]
J Agric Food Chem. 2010 Mar 24;58(6):3847-54.
Ginger, the rhizome of Zingiber officinale , is a traditional medicine with a carminative effect and antinausea, anti-inflammatory, and anticarcinogenic properties. This study examined the growth inhibitory effects of [8]-shogaol, one of the pungent phenolic compounds in ginger, on human leukemia HL-60 cells. It demonstrated that [8]-shogaol was able to induce apoptosis in a time- and concentration-dependent manner. Treatment with [8]-shogaol caused a rapid loss of mitochondrial transmembrane potential, stimulation of reactive oxygen species (ROS) production, release of mitochondrial cytochrome c into cytosol, and subsequent induction of procaspase-9 and procaspase-3 processing. Taken together, these results suggest for the first time that ROS production and depletion of glutathione that contributed to [8]-shogaol-induced apoptosis in HL-60 cells.
Characterization of thiol-conjugated metabolites of ginger components shogaols in mouse and human urine and modulation of the glutathione levels in cancer cells by [6]-shogaol.[Pubmed:23322393]
Mol Nutr Food Res. 2013 Mar;57(3):447-58.
SCOPE: Shogaols, a series of major constituents in dried ginger with the most abundant being [6]-, [8]-, and [10]-shogaols, show much higher anticancer potencies than gingerols. Previously, we reported the mercapturic acid pathway as a major metabolic route for [6]-shogaol in mice. However, it is still unclear how the side chain length affects the metabolism of shogaols and how shogaols are metabolized in humans. METHODS AND RESULTS: We first investigate the metabolism of [10]-shogaol in mouse urine, and then investigate the biotransformation of shogaols in human urine. Our results show that eight major thiol-conjugated metabolites of [10]-shogaol were detected in mouse urine, while six major thiol-conjugated metabolites of [6]-shogaol, two thiol-conjugated metabolites of [8]-shogaol, and two thiol-conjugated metabolites of [10]-shogaol were detected in urine collected from human after drinking ginger tea, using LC/ESI-MS/MS. Our results clearly indicate the mercapturic acid pathway is a major metabolic route for [10]-shogaol in mice and for shogaols in human. Furthermore, we also investigated the regulation of glutathione (GSH) by [6]-shogaol in human colon cancer cells HCT-116. Our results show [6]-shogaol, after initially depleting glutathione levels, can subsequently restore and increase GSH levels over time. CONCLUSION: Shogaols are metabolized extensively in mouse and human to form thiol-conjugated metabolites and GSH might play an important role in the cancer-preventive activity of ginger.


