Alisol C monoacetateCAS# 26575-93-9 |
Quality Control & MSDS
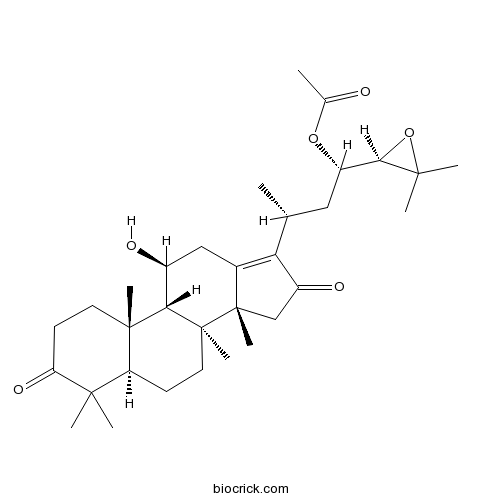
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 26575-93-9 | SDF | Download SDF |
| PubChem ID | 14036813 | Appearance | White powder |
| Formula | C32H48O6 | M.Wt | 528.73 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Synonyms | 23-O-Acetylalisol C; Alisol C monoacetate | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1S,3R)-1-[(2R)-3,3-dimethyloxiran-2-yl]-3-[(5R,8S,9S,10S,11S,14R)-11-hydroxy-4,4,8,10,14-pentamethyl-3,16-dioxo-2,5,6,7,9,11,12,15-octahydro-1H-cyclopenta[a]phenanthren-17-yl]butyl] acetate | ||
| SMILES | CC(CC(C1C(O1)(C)C)OC(=O)C)C2=C3CC(C4C5(CCC(=O)C(C5CCC4(C3(CC2=O)C)C)(C)C)C)O | ||
| Standard InChIKey | KOOCQNIPRJEMDH-QSKXMHMESA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Alisol C monoacetate(Alisol C 23-acetate) has antibacterial activity. |
| Targets | Antifection |
| In vitro | A new triterpenoid from Alisma orientale and their antibacterial effect.[Pubmed: 23212633 ]Arch. Pharm. Res., 2012, 35(11):1919-26.A new triterpenoid, named alisol Q 23-acetate, as well as fourteen known terpenes, alisol B 23-acetate (2), alisol B (3), alismol (4), 10-O-methyl-alismoxide (5), alismoxide (6), 11-deoxyalisol C (7), 13β,17β-epoxyalisol B 23-acetate (8), 4β,12-dihydroxyguaian-6,10-diene (9), alisol C 23-acetate (Alisol C monoacetate,10), alisolide (11), 16β-methoxyalisol B monoacetate (12), alisol A (13), 16β-hydroxyalisol B 23-acetate (14), alisol A 24-acetate (15) were isolated from the rhizomes of Alisma orientale.
|
| Structure Identification | Chem.Pharm. Bull.,1970,18(7):1369-84.Biological-Active Triterpenes of Alismatis Rhizoma. IV. The Structures of Alisol B, Alisol B Monoacetate and Alisol C Monoacetate-Some Reactions of the α-Hydroxy Epoxide of the Alisol B Derivatives[Reference: WebLink]Studies on the structures of alisol B, alisol B monoacetate and Alisol C monoacetate, the new biological-active triterpenes of Alismatis Rhizoma, are reported. |

Alisol C monoacetate Dilution Calculator

Alisol C monoacetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8913 mL | 9.4566 mL | 18.9132 mL | 37.8265 mL | 47.2831 mL |
| 5 mM | 0.3783 mL | 1.8913 mL | 3.7826 mL | 7.5653 mL | 9.4566 mL |
| 10 mM | 0.1891 mL | 0.9457 mL | 1.8913 mL | 3.7826 mL | 4.7283 mL |
| 50 mM | 0.0378 mL | 0.1891 mL | 0.3783 mL | 0.7565 mL | 0.9457 mL |
| 100 mM | 0.0189 mL | 0.0946 mL | 0.1891 mL | 0.3783 mL | 0.4728 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Alisol C 23-acetate, a natural product extracted from Alisma orientale, can significantly and strongly inhibit DTH response after oral administration.
References:
[1]. Lee JH, et al. The rhizomes of Alisma orientale and alisol derivatives inhibit allergic response and experimental atopic dermatitis. Biol Pharm Bull. 2012;35(9):1581-7.
- Z-D-Glu-OMe
Catalog No.:BCC2773
CAS No.:26566-11-0
- 3-Hydroxycatalponol
Catalog No.:BCN5146
CAS No.:265644-24-4
- 2,3-Dihydroxy-12-oleanen-28-oic acid
Catalog No.:BCN5145
CAS No.:26563-68-8
- 8-Phenyloctanoic acid
Catalog No.:BCC8790
CAS No.:26547-51-3
- Apiin
Catalog No.:BCN2311
CAS No.:26544-34-3
- (-)-Hinokinin
Catalog No.:BCN3227
CAS No.:26543-89-5
- Ryuvidine
Catalog No.:BCC7432
CAS No.:265312-55-8
- GW 7647
Catalog No.:BCC7150
CAS No.:265129-71-3
- Fosaprepitant dimeglumine salt
Catalog No.:BCC4954
CAS No.:265121-04-8
- Taiwanhomoflavone A
Catalog No.:BCN6853
CAS No.:265120-00-1
- N-[Bis(methylthio)methylene]- p-toluenesulfonamide
Catalog No.:BCC9069
CAS No.:2651-15-2
- Methyleugenolglycol
Catalog No.:BCN6562
CAS No.:26509-45-5
- Alisol B 23-acetate
Catalog No.:BCN1243
CAS No.:26575-95-1
- 5-Acetoacetlamino benzimdazolone
Catalog No.:BCC8725
CAS No.:26576-46-5
- Harmalacidine
Catalog No.:BCN8033
CAS No.:26579-69-1
- Dehydrocrenatine
Catalog No.:BCN5147
CAS No.:26585-13-7
- Crenatine
Catalog No.:BCN5148
CAS No.:26585-14-8
- SCH 202676 hydrobromide
Catalog No.:BCC7049
CAS No.:265980-25-4
- Z-D-Ala-OH
Catalog No.:BCC3059
CAS No.:26607-51-2
- 3-Ethoxyandrosta-3,5-dien-17β-ol
Catalog No.:BCC8631
CAS No.:26614-48-2
- Zotepine
Catalog No.:BCC7838
CAS No.:26615-21-4
- Fmoc-β-Homo-Met-OH
Catalog No.:BCC2630
CAS No.:266359-48-2
- Reparixin
Catalog No.:BCC1885
CAS No.:266359-83-5
- Reparixin L-lysine salt
Catalog No.:BCC1886
CAS No.:266359-93-7
A new triterpenoid from Alisma orientale and their antibacterial effect.[Pubmed:23212633]
Arch Pharm Res. 2012 Nov;35(11):1919-26.
A new triterpenoid, named alisol Q 23-acetate, as well as fourteen known terpenes, alisol B 23-acetate (2), alisol B (3), alismol (4), 10-O-methyl-alismoxide (5), alismoxide (6), 11-deoxyalisol C (7), 13beta,17beta-epoxyalisol B 23-acetate (8), 4beta,12-dihydroxyguaian-6,10-diene (9), alisol C 23-acetate (10), alisolide (11), 16beta-methoxyalisol B monoacetate (12), alisol A (13), 16beta-hydroxyalisol B 23-acetate (14), alisol A 24-acetate (15) were isolated from the rhizomes of Alisma orientale. The structures of compounds (1-15) were identified based on 1D and 2D NMR, including (1)H-(1)H COSY, HSQC, HMBC and NOESY spectroscopic analyses. Among these isolates, antibacterial effect of compounds 2, 3, 10, and 15, major constituents of A. orientale was examined. The MIC values of compounds 2, 10, and 15 were 5-10 betag/mL against eight antibiotic resistant strains, which were lower than those from the positive controls (MICs of chloramphenicol and ampicillin were 5-80 mug/mL). Therefore, compounds 2, 10 and 15 exhibited the potent antibacterial activity.


