(-)-HinokininCAS# 26543-89-5 |
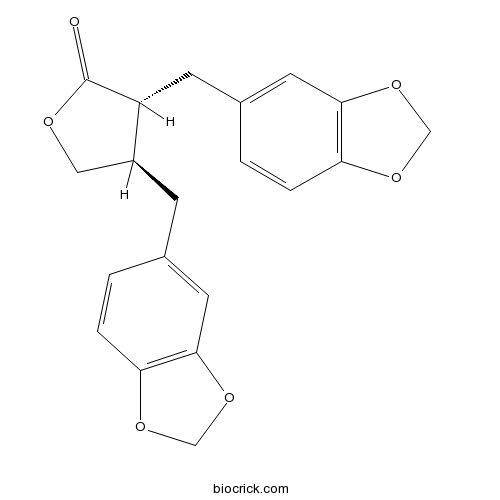
- Heliobuphthalmin lactone
Catalog No.:BCX0198
CAS No.:580-73-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 26543-89-5 | SDF | Download SDF |
| PubChem ID | 442879 | Appearance | Pale yellow-brown gelatinous |
| Formula | C20H18O6 | M.Wt | 354.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Synonyms | Cubebinolide; Dihydroisohibalactone | ||
| Solubility | Insoluble in water | ||
| Chemical Name | (3R,4R)-3,4-bis(1,3-benzodioxol-5-ylmethyl)oxolan-2-one | ||
| SMILES | C1C(C(C(=O)O1)CC2=CC3=C(C=C2)OCO3)CC4=CC5=C(C=C4)OCO5 | ||
| Standard InChIKey | DDWGQGZPYDSYEL-LSDHHAIUSA-N | ||
| Standard InChI | InChI=1S/C20H18O6/c21-20-15(6-13-2-4-17-19(8-13)26-11-24-17)14(9-22-20)5-12-1-3-16-18(7-12)25-10-23-16/h1-4,7-8,14-15H,5-6,9-11H2/t14-,15+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. (-)-Hinokinin has anti-genotoxic and anticarcinogenic potential. 2. (-)-Hinokinin may serve as a tool to develop new therapeutic drugs for attention deficit hyperactivity disorder. 3. (-)-Hinokinin is an anti-chagasic drug, has no mutagenic effects in animal cell and bacterial systems, anxiety that target the DAT, NET, and GAT-1 transporters. |

(-)-Hinokinin Dilution Calculator

(-)-Hinokinin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8217 mL | 14.1084 mL | 28.2167 mL | 56.4334 mL | 70.5418 mL |
| 5 mM | 0.5643 mL | 2.8217 mL | 5.6433 mL | 11.2867 mL | 14.1084 mL |
| 10 mM | 0.2822 mL | 1.4108 mL | 2.8217 mL | 5.6433 mL | 7.0542 mL |
| 50 mM | 0.0564 mL | 0.2822 mL | 0.5643 mL | 1.1287 mL | 1.4108 mL |
| 100 mM | 0.0282 mL | 0.1411 mL | 0.2822 mL | 0.5643 mL | 0.7054 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ryuvidine
Catalog No.:BCC7432
CAS No.:265312-55-8
- GW 7647
Catalog No.:BCC7150
CAS No.:265129-71-3
- Fosaprepitant dimeglumine salt
Catalog No.:BCC4954
CAS No.:265121-04-8
- Taiwanhomoflavone A
Catalog No.:BCN6853
CAS No.:265120-00-1
- N-[Bis(methylthio)methylene]- p-toluenesulfonamide
Catalog No.:BCC9069
CAS No.:2651-15-2
- Methyleugenolglycol
Catalog No.:BCN6562
CAS No.:26509-45-5
- Z-Gln-OH
Catalog No.:BCC2783
CAS No.:2650-64-8
- Nandrolone laurate
Catalog No.:BCC9088
CAS No.:26490-31-3
- Clovanediol diacetate
Catalog No.:BCN5144
CAS No.:2649-68-5
- Clovanediol
Catalog No.:BCN5143
CAS No.:2649-64-1
- Cyclo(D-Phe-L-Pro)
Catalog No.:BCN4011
CAS No.:26488-24-4
- 6-Deoxyisojacareubin
Catalog No.:BCN7723
CAS No.:26486-92-0
- Apiin
Catalog No.:BCN2311
CAS No.:26544-34-3
- 8-Phenyloctanoic acid
Catalog No.:BCC8790
CAS No.:26547-51-3
- 2,3-Dihydroxy-12-oleanen-28-oic acid
Catalog No.:BCN5145
CAS No.:26563-68-8
- 3-Hydroxycatalponol
Catalog No.:BCN5146
CAS No.:265644-24-4
- Z-D-Glu-OMe
Catalog No.:BCC2773
CAS No.:26566-11-0
- Alisol C monoacetate
Catalog No.:BCN2345
CAS No.:26575-93-9
- Alisol B 23-acetate
Catalog No.:BCN1243
CAS No.:26575-95-1
- 5-Acetoacetlamino benzimdazolone
Catalog No.:BCC8725
CAS No.:26576-46-5
- Harmalacidine
Catalog No.:BCN8033
CAS No.:26579-69-1
- Dehydrocrenatine
Catalog No.:BCN5147
CAS No.:26585-13-7
- Crenatine
Catalog No.:BCN5148
CAS No.:26585-14-8
- SCH 202676 hydrobromide
Catalog No.:BCC7049
CAS No.:265980-25-4
The lignan (-)-hinokinin displays modulatory effects on human monoamine and GABA transporter activities.[Pubmed:24112084]
J Nat Prod. 2013 Oct 25;76(10):1889-95.
The neurotransmitter transporters of the SLC6 family play critical roles in the regulation of neurotransmission and are the primary targets of therapeutic agents used to treat clinical disorders involving compromised neurotransmitter signaling. The dopamine and norepinephrine transporters have been implicated in clinical disorders such as attention deficit hyperactivity disorder (ADHD) and substance abuse. The GABA transporters (GATs) serve as a target for anxiolytic, antidepressant, and antiepileptic therapies. In this work, the interaction with neurotransmitter transporters was characterized for a derivative of the lignan (-)-cubebin (1), namely, (-)-Hinokinin (2). Using in vitro pharmacological assays, 2 selectively inhibited the human dopamine and norepinephrine transporters, in a noncompetitive manner possibly mediated by binding to a novel site within the transporters, and displayed low affinity for the serotonin transporter. Compound 2 also specifically inhibited the GAT-1 GABA transporter subtype. Compound 2 is not a substrate of the carriers as it had no effect on the efflux of either of the neurotransmitters investigated. This compound is inactive toward glutamate and glycine transporters. These results suggest that 2 may serve as a tool to develop new therapeutic drugs for ADHD and anxiety that target the DAT, NET, and GAT-1 transporters.
Chemopreventive effects of (-)-hinokinin against 1,2-dimethylhydrazine-induced genotoxicity and preneoplastic lesions in rat colon.[Pubmed:25297647]
J Nat Prod. 2014 Oct 24;77(10):2312-5.
(-)-Hinokinin (1) is a dibenzylbutyrolactone lignan obtained by the partial synthesis of (-)-cubebin. This study reports the antigenotoxic and anticarcinogenic potential of 1 by the comet and aberrant crypt focus assays in the peripheral blood and colon of 4-5-week-old Wistar rats, respectively. The rats were exposed to 1,2-dimethylhydrazine (40 mg/kg) and were treated by gavage with doses of 10, 20, and 40 mg/kg of 1. The results showed that the dose of 40 mg/kg was neither genotoxic nor carcinogenic. In the comet assay, all 1 doses displayed antigenotoxic effects. In addition, this compound (20 and 40 mg/kg) exhibited an anticarcinogenic effect in the aberrant crypt focus assay.
Evaluation of the in vivo therapeutic properties of (-)-cubebin and (-)-hinokinin against Trypanosoma cruzi.[Pubmed:23274812]
Exp Parasitol. 2013 Apr;133(4):442-6.
Even though the Chagas' disease, caused by the protozoan Trypanosoma cruzi, was described 100years ago by Carlos Chagas, it still represents a major public health concern and is found in 18 developing countries in South and Central America. In Brazil, Benznidazole (Rochagan) is the only drug with trypanocidal activity available in the market, despite its several side effects and limited efficacy in the chronic phase of the infection. In view of the need for new substances displaying biological activity against T. cruzi, there has been growing interest in research toward the attainment of compounds capable of acting on the parasite while being devoid of serious side effects. In this context, this study aims to evaluate the in vivo therapeutic activity of dibenzylbutyrolactone lignans (-)-cubebin and (-)-Hinokinin during the acute phase of infection by T. cruzi. As a study criterion, animals with acute parasitemia were investigated by tissue morphometric analysis. There was significant parasitemia reduction in the groups of animals treated with (-)-cubebin or (-)-hinokin oral administration, compared to the negative control. Values close to those of the uninfected control were found in the groups treated with (-)-cubebin and (-)-Hinokinin via kariometry, showing that there was positive cellular response compared to the infected control.
Mutagenicity and antimutagenicity of (-)-hinokinin a trypanosomicidal compound measured by Salmonella microsome and comet assays.[Pubmed:23114276]
BMC Complement Altern Med. 2012 Oct 31;12:203.
BACKGROUND: The dibenzylbutyrolactone lignan (-)-Hinokinin (HK) was derived by partial synthesis from (-)-cubebin, isolated from the dry seeds of the pepper, Piper cubeba. Considering the good trypanosomicidal activity of HK and recalling that natural products are promising starting points for the discovery of novel potentially therapeutic agents, the aim of the present study was to investigate the (anti) mutagenic genotoxic activities of HK. METHODS: The mutagenic genotoxic activities were evaluated by the Ames test on Salmonella typhimurium strains TA98, TA97a, TA100 and TA102, and the comet assay, so as to assess the safe use of HK in the treatment of Chagas' disease. The antimutagenic antigenotoxic potential of HK were also tested against the mutagenicity of a variety of direct and indirect acting mutagens, such as 4- nitro-o-phenylenediamine (NOPD), sodium azide (SA), mitomycin C (MMC), benzo[a]pyrene (B[a]P), aflatoxin B1 (AFB1), 2-aminoanthracene (2-AA) and 2-aminofluorene (2-AF), by the Ames test, and doxorubicin (DXR) by the comet assay. RESULTS: The mutagenicitygenotoxicity tests showed that HK did not induce any increase in the number of revertants or extent of DNA damage, demonstrating the absence of mutagenic and genotoxic activities. On the other hand, the results on the antimutagenic potential of HK showed a strong inhibitory effect against some direct and indirect-acting mutagens. CONCLUSIONS: Regarding the use of HK as an antichagasic drug, the absence of mutagenic effects in animal cell and bacterial systems is encouraging. In addition, HK may be a new potential antigenotoxic antimutagenic agent from natural sources. However, the protective activity of HK is not general and varies with the type of DNA damage-inducing agent used.


