AlloimperatorinCAS# 642-05-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 642-05-7 | SDF | Download SDF |
| PubChem ID | 69502 | Appearance | Powder |
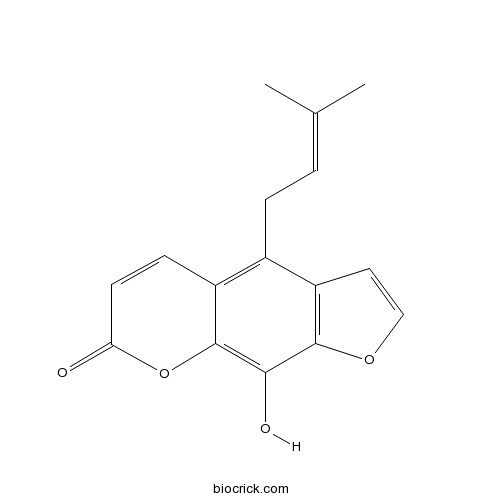
| Formula | C16H14O4 | M.Wt | 270.3 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 9-hydroxy-4-(3-methylbut-2-enyl)furo[3,2-g]chromen-7-one | ||
| SMILES | CC(=CCC1=C2C=CC(=O)OC2=C(C3=C1C=CO3)O)C | ||
| Standard InChIKey | KDXVVZMYSLWJMA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H14O4/c1-9(2)3-4-10-11-5-6-13(17)20-16(11)14(18)15-12(10)7-8-19-15/h3,5-8,18H,4H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Alloimperatorin photosensitizes efficiently the hemolysis of erythrocytes. 2. Alloimperatorin exhibits in vitro antitumor activity in HL-60 acute myeloid leukemia cancer cells via inducing apoptosis, cell cycle disruption and inhibition of cell migration. |

Alloimperatorin Dilution Calculator

Alloimperatorin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6996 mL | 18.498 mL | 36.9959 mL | 73.9919 mL | 92.4898 mL |
| 5 mM | 0.7399 mL | 3.6996 mL | 7.3992 mL | 14.7984 mL | 18.498 mL |
| 10 mM | 0.37 mL | 1.8498 mL | 3.6996 mL | 7.3992 mL | 9.249 mL |
| 50 mM | 0.074 mL | 0.37 mL | 0.7399 mL | 1.4798 mL | 1.8498 mL |
| 100 mM | 0.037 mL | 0.185 mL | 0.37 mL | 0.7399 mL | 0.9249 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Barbinervic acid
Catalog No.:BCN4061
CAS No.:64199-78-6
- Senaetnine
Catalog No.:BCN2127
CAS No.:64191-69-1
- Zanthobungeanine
Catalog No.:BCN6685
CAS No.:64190-94-9
- Z-Hyp-Ome
Catalog No.:BCC3258
CAS No.:64187-48-0
- trans-2,3-Dihydro-3-hydroxyeuparin
Catalog No.:BCN6922
CAS No.:64185-57-5
- 30-Oxolupeol
Catalog No.:BCN6673
CAS No.:64181-07-3
- 5-Acetyl-2-(1-hydroxy-1-methylethyl)benzofuran
Catalog No.:BCN7488
CAS No.:64165-99-7
- Echinoynethiophene A
Catalog No.:BCN4183
CAS No.:64165-98-6
- Nilotinib(AMN-107)
Catalog No.:BCC3643
CAS No.:641571-10-0
- Sedanolide
Catalog No.:BCN8338
CAS No.:6415-59-4
- Longistylin C
Catalog No.:BCN4182
CAS No.:64125-60-6
- Di-O-methylcrenatin
Catalog No.:BCN4608
CAS No.:64121-98-8
- Akuammigine
Catalog No.:BCN4607
CAS No.:642-17-1
- Alstonine
Catalog No.:BCN4606
CAS No.:642-18-2
- L-Quebrachitol
Catalog No.:BCN2727
CAS No.:642-38-6
- Antiarol
Catalog No.:BCN4185
CAS No.:642-71-7
- CGP 12177 hydrochloride
Catalog No.:BCC6949
CAS No.:64208-32-8
- Thalirugidine
Catalog No.:BCN7706
CAS No.:64215-95-8
- Atracurium oxalate
Catalog No.:BCC8837
CAS No.:64228-78-0
- L189
Catalog No.:BCC7707
CAS No.:64232-83-3
- Taraxasterol acetate
Catalog No.:BCN4184
CAS No.:6426-43-3
- Glutinol acetate
Catalog No.:BCN6675
CAS No.:6426-44-4
- Boc-N-Me-Tyr(Bzl)-OH
Catalog No.:BCC3356
CAS No.:64263-81-6
- Kielcorin
Catalog No.:BCN7637
CAS No.:64280-48-4
Photohemolysis Sensitized by the Furocoumarin Derivative Alloimperatorin and its Hydroperoxide Photooxidation Product.[Pubmed:24117477]
Photochem Photobiol. 2014 Jan;90(1):162-70.
The dark and photosensitized effects of Alloimperatorin methyl ether 1 (hereafter simply alloimperatorin) and its photooxygenation product Alloimperatorin hydroperoxide 2 were investigated on human erythrocytes. The results reveal that the furocoumarin 1 photosensitizes efficiently the hemolysis of erythrocytes. The rate of photohemolysis increases on raising the temperature of the postirradiated incubation from 4 degrees C to 37 degrees C. Thermal activation of the photohemolysis and inhibition by 2,6-di-tert-butyl-p-cresol (BHT) suggest that the furocoumarin 1 photosensitizes lipid peroxidation, increasing permeability in the erythrocyte membrane. The hydroperoxide 2 induces dark and photosensitized hemolysis more efficiently than the furocoumarin 1. The rate of hemolysis induced by 2 increases with the incubation temperature and decreases in the presence of tert-butanol and BHT. The hydroperoxide 2 photosensitizes the formation of lipid peroxidation products as shown by the reaction with thiobarbituric acid. This process is diminished by BHT. Our data imply that the photohemolysis sensitized by the furocoumarin 1 is caused by the in situ-formed photooxygenation product 2. Such hydroperoxides are potent hemolytic agents in the dark and especially on photosensitization.


