Ampelopsin ACAS# 130608-11-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 130608-11-6 | SDF | Download SDF |
| PubChem ID | 182999 | Appearance | Powder |
| Formula | C28H22O7 | M.Wt | 470.5 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
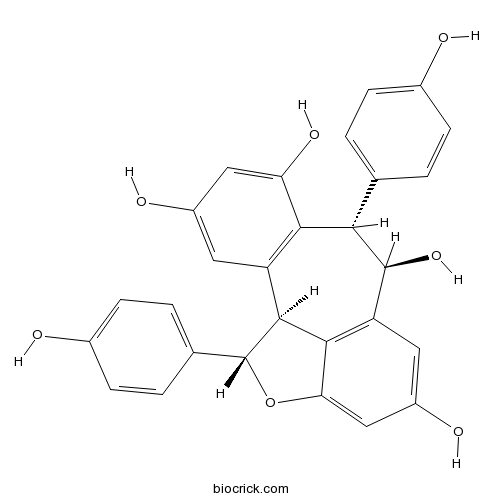
| Chemical Name | (1S,8S,9R,16S)-8,16-bis(4-hydroxyphenyl)-15-oxatetracyclo[8.6.1.02,7.014,17]heptadeca-2(7),3,5,10(17),11,13-hexaene-4,6,9,12-tetrol | ||
| SMILES | C1=CC(=CC=C1C2C(C3=C4C(C(OC4=CC(=C3)O)C5=CC=C(C=C5)O)C6=C2C(=CC(=C6)O)O)O)O | ||
| Standard InChIKey | LHUHHURKGTUZHU-QWMXJGQVSA-N | ||
| Standard InChI | InChI=1S/C28H22O7/c29-15-5-1-13(2-6-15)23-24-19(9-17(31)11-21(24)33)26-25-20(27(23)34)10-18(32)12-22(25)35-28(26)14-3-7-16(30)8-4-14/h1-12,23,26-34H/t23-,26-,27-,28+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ampelopsin A Dilution Calculator

Ampelopsin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1254 mL | 10.627 mL | 21.254 mL | 42.508 mL | 53.135 mL |
| 5 mM | 0.4251 mL | 2.1254 mL | 4.2508 mL | 8.5016 mL | 10.627 mL |
| 10 mM | 0.2125 mL | 1.0627 mL | 2.1254 mL | 4.2508 mL | 5.3135 mL |
| 50 mM | 0.0425 mL | 0.2125 mL | 0.4251 mL | 0.8502 mL | 1.0627 mL |
| 100 mM | 0.0213 mL | 0.1063 mL | 0.2125 mL | 0.4251 mL | 0.5313 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Aesculioside D
Catalog No.:BCN9551
CAS No.:254896-66-7
- cis-Abienol
Catalog No.:BCN9550
CAS No.:17990-16-8
- 7-(3'-Carboxybutoxy)coumarin
Catalog No.:BCN9549
CAS No.:16851-01-7
- Buxifoliadine H
Catalog No.:BCN9548
CAS No.:263007-72-3
- Coryximine
Catalog No.:BCN9547
CAS No.:127460-61-1
- 6-Methoxykaempferol 3-O-galactoside
Catalog No.:BCN9546
CAS No.:72945-43-8
- Santin
Catalog No.:BCN9545
CAS No.:27782-63-4
- 5,5'-Dihydroxy-3,8,3',4'-tetramethoxy-6,7-methylenedioxyflavone
Catalog No.:BCN9544
CAS No.:82668-96-0
- 4"-O-Acetylastilbin
Catalog No.:BCN9543
CAS No.:1298135-49-5
- (1R,6R,9R)-6,9,11-Trihydroxy-4,7-megastigmadien-3-one 11-O-glucoside
Catalog No.:BCN9542
CAS No.:289914-68-7
- Atalafoline
Catalog No.:BCN9541
CAS No.:107259-49-4
- Atalaphylline
Catalog No.:BCN9540
CAS No.:28233-35-4
- Kaempferol 3,5-dimethyl ether
Catalog No.:BCN9553
CAS No.:1486-65-3
- Acersaponin I
Catalog No.:BCN9554
CAS No.:1257940-29-6
- 2,3-Dihydro-6-methylginkgetin
Catalog No.:BCN9555
CAS No.:1013649-09-6
- 3-Formylcarbazole
Catalog No.:BCN9556
CAS No.:51761-07-0
- Vitisin A
Catalog No.:BCN9557
CAS No.:142449-89-6
- Vanicoside B
Catalog No.:BCN9558
CAS No.:155179-21-8
- Sonderianol
Catalog No.:BCN9559
CAS No.:85563-65-1
- Isoaesculioside D
Catalog No.:BCN9560
CAS No.:1184581-59-6
- Aesculioside C
Catalog No.:BCN9561
CAS No.:254896-65-6
- Quercetin 3,5,3'-trimethyl ether
Catalog No.:BCN9562
CAS No.:13459-09-1
- 3-O-Methylellagic acid 4-O-rhamnoside
Catalog No.:BCN9563
CAS No.:639089-97-7
- Adoxoside
Catalog No.:BCN9564
CAS No.:42830-26-2
Control of Reactive Oxygen Species for the Prevention of Parkinson's Disease: The Possible Application of Flavonoids.[Pubmed:32635299]
Antioxidants (Basel). 2020 Jul 3;9(7). pii: antiox9070583.
Oxidative stress reflects an imbalance between the production of reactive oxygen species (ROS) and antioxidant defense systems, and it can be associated with the pathogenesis and progression of neurodegenerative diseases such as multiple sclerosis, stroke, and Parkinson's disease (PD). The application of antioxidants, which can defend against oxidative stress, is able to detoxify the reactive intermediates and prevent neurodegeneration resulting from excessive ROS production. There are many reports showing that numerous flavonoids, a large group of natural phenolic compounds, can act as antioxidants and the application of flavonoids has beneficial effects in the adult brain. For instance, it is well known that the long-term consumption of the green tea-derived flavonoids catechin and epigallocatechin gallate (EGCG) can attenuate the onset of PD. Also, flavonoids such as Ampelopsin And pinocembrin can inhibit mitochondrial dysfunction and neuronal death through the regulation of gene expression of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway. Additionally, it is well established that many flavonoids exhibit anti-apoptosis and anti-inflammatory effects through cellular signaling pathways, such as those involving (ERK), glycogen synthase kinase-3beta (GSK-3beta), and (Akt), resulting in neuroprotection. In this review article, we have described the oxidative stress involved in PD and explained the therapeutic potential of flavonoids to protect the nigrostriatal DA system, which may be useful to prevent PD.
Screening of Natural Stilbene Oligomers from Vitis vinifera for Anticancer Activity on Human Hepatocellular Carcinoma Cells.[Pubmed:32492881]
Antioxidants (Basel). 2020 Jun 1;9(6). pii: antiox9060469.
The characterization of bioactive resveratrol oligomers extracted from Vitis vinifera canes has been recently reported. Here, we screened six of these compounds (Ampelopsin A, trans-epsilon-viniferin, hopeaphenol, isohopeaphenol, R2-viniferin, and R-viniferin) for their cytotoxic activity to human hepatocellular carcinoma (HCC) cell lines p53 wild-type HepG2 and p53-null Hep3B. The cytotoxic efficacy depended on the cell line. R2-viniferin was the most toxic stilbene in HepG2, with inhibitory concentration 50 (IC50) of 9.7 +/- 0.4 microM at 72 h, 3-fold lower than for resveratrol, while Hep3B was less sensitive (IC50 of 47.8 +/- 2.8 microM). By contrast, hopeaphenol (IC50 of 13.1 +/- 4.1 microM) and isohopeaphenol (IC50 of 26.0 +/- 3.0 microM) were more toxic to Hep3B. Due to these results, and because it did not exert a large cytotoxicity in HH4 non-transformed hepatocytes, R2-viniferin was selected to investigate its mechanism of action in HepG2. The stilbene tended to arrest cell cycle at G2/M, and it also increased intracellular reactive oxygen species (ROS), caspase 3 activity, and the ratio of Bax/Bcl-2 proteins, indicative of apoptosis. The distinctive toxicity of R2-viniferin on HepG2 encourages research into the underlying mechanism to develop the oligostilbene as a therapeutic agent against HCC with a particular genetic background.
Grape Cane Extracts as Multifunctional Rejuvenating Cosmetic Ingredient: Evaluation of Sirtuin Activity, Tyrosinase Inhibition and Bioavailability Potential.[Pubmed:32397228]
Molecules. 2020 May 8;25(9). pii: molecules25092203.
Grape canes are waste biomass of viticulture containing bioactive polyphenols valuable in cosmetics. Whereas several studies reported the cosmetic activities of E-resveratrol, only few described the potential of E-epsilon-viniferin, the second major constituent of grape cane extracts (GCE), and none of them investigated GCE as a natural blend of polyphenols for cosmetic applications. In this study, we considered the potential of GCE from polyphenol-rich grape varieties as multifunctional cosmetic ingredients. HPLC analysis was performed to quantify major polyphenols in GCE i.e., catechin, epicatechin, E-resveratrol, E-piceatannol, Ampelopsin A, E-epsilon-viniferin, hopeaphenol, isohopeaphenol, E-miyabenol C and E-vitisin B from selected cultivars. Skin whitening potential through tyrosinase inhibition assay and the activation capacity of cell longevity protein (SIRT1) of GCE were compared to pure E-resveratrol and E-epsilon-viniferin. Drug-likeness of GCE polyphenols were calculated, allowing the prediction of skin permeability and bioavailability. Finally, the present data enabled the consideration of GCE from polyphenol-rich varieties as multifunctional cosmetic ingredients in accordance with green chemistry practices.
A stilbene dimer and flavonoids from the aerial parts of Chromolaena odorata with proprotein convertase subtilisin/kexin type 9 expression inhibitory activity.[Pubmed:32335358]
Bioorg Chem. 2020 Jun;99:103869.
Investigation of components of the chloroform-soluble and ethyl acetate-soluble extracts of the aerial parts of Chromolaena odorata L. selected by PCSK9 mRNA expression monitoring assay in HepG2 cells led to the isolation of a new stilbene dimer, (+)-8b-epi-Ampelopsin A (1), and 30 known compounds (2-31). The structures of the isolates were established by interpretation of NMR spectroscopic data and the stereochemistry of the new stilbene (1) was proposed based on ECD and NMR calculations. Among the isolates, 1, 5,6,7,4'-tetramethoxyflavanone (6), 5,6,7,3',4'-pentamethoxyflavanone (7), acacetin (18), and uridine (21) were found to inhibit PCSK9 mRNA expression with IC50 values of 20.6, 21.4, 31.7, 15.0, and 13.7 microM, respectively. Furthermore, the most abundant isolate among the selected compounds, 6, suppressed PCSK9 and low-density lipoprotein receptor protein expression in addition to downregulating the mRNA expression of HNF-1alpha.
Inhibition of the type III secretion system of Pseudomonas syringae pv. tomato DC3000 by resveratrol oligomers identified in Vitis vinifera L.[Pubmed:31994325]
Pest Manag Sci. 2020 Jul;76(7):2294-2303.
BACKGROUND: The bacterial type III secretion system (T3SS) is one of the virulence determinants of Gram-negative bacteria through which various effector and virulence proteins are translocated into host cells. RESULTS: We constructed an assay system to screen inhibitors of hrpA gene expression (a structural gene of Hrp pili) in Pseudomonas syringae pv. tomato DC3000. In a plant extract library screening, the root extract of Vitis vinifera L. displayed the most prominent activity. Three resveratrol oligomers, hopeaphenol, isohopeaphenol and Ampelopsin A, were identified in grapevine root extract, which significantly reduced the transcription levels of the hrpA, hrpL and hopP1 genes without growth retardation. Additional resveratrol derivatives identified in other plant extracts were also examined for their inhibitory effect on hrpA expression. Another resveratrol oligomer, kobophenol A, also inhibited the transcription of the hrpA gene and other T3SS-related genes, while resveratrol monomers (resveratrol and piceatannol) were not effective. The severity of bacterial specks was reduced by each hopeaphenol, isohopeaphenol and Ampelopsin A treatment. CONCLUSION: These results show the potential of resveratrol derivatives as anti-virulence agents for the control of plant diseases.
Ampelopsin alleviates sevoflurane-induced cognitive dysfunction by mediating NF-kappaB pathway in aged rats.[Pubmed:31902108]
Genes Genomics. 2020 Apr;42(4):361-369.
BACKGROUND: Cancer-induced bone pain (CIBP) is the pain caused by bone metastasis from malignant tumors, and the largest source of pain for cancer patients. miR-300 is an important miRNA in cancer. It has been shown that miR-300 regulates tumorigenesis of various tumors. PURPOSE: This study aims to investigate the role of miR-300 in CIBP and its underlying molecular mechanisms in vitro and in vivo. METHODS: We constructed CIBP model in rats and investigated the mechanism through which miR-300 affects CIBP. We first examined expression level of miR-300 in CIBP rats and then tested the effect of its overexpression. Next, we identified the target of miR-300 using TargetScan analysis and double luciferase assay. Finally, we studied genetic interactions between miR-300 and its target and their roles in CIBP. RESULTS: We found that miR-300 was downregulated in CIBP rats. Overexpression of miR-300 significantly attenuated cancer-induced neuropathic pain (p < 0.01). Furthermore, TargetScan analysis and double luciferase assay show High Mobility Group Box 1 (HMGB1) is a target of miR-300. Notably, HMGB1 is overexpressed in CIBP rats, while up-regulation of miR-300 significantly suppresses expression of HMGB1 (p < 0.01). Moreover, knockdown of HMGB1 by siRNA significantly relieves cancer-induced neuropathic pain in rats (p < 0.01). On the other hand, HMGB1 overexpression partially blocked the effect of miR-300 on cancer-induced nerve pain. CONCLUSION: miR-300 relieves cancer-induced neuropathic pain by inhibiting HMGB1 expression. These results may be beneficial for the treatment of CIBP in clinical practice.


