cis-AbienolCAS# 17990-16-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 17990-16-8 | SDF | Download SDF |
| PubChem ID | 643723 | Appearance | Oil |
| Formula | C20H34O | M.Wt | 290.5 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
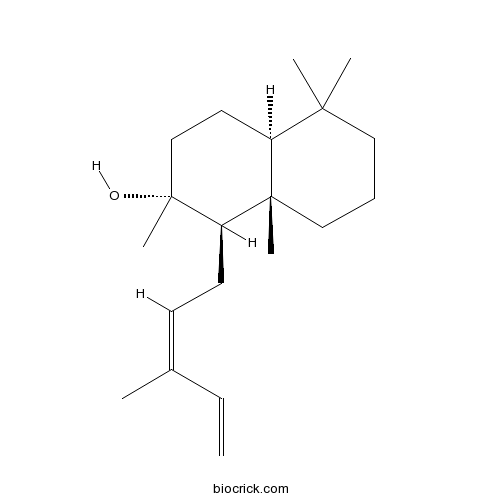
| Chemical Name | (1R,2R,4aS,8aS)-2,5,5,8a-tetramethyl-1-[(2Z)-3-methylpenta-2,4-dienyl]-3,4,4a,6,7,8-hexahydro-1H-naphthalen-2-ol | ||
| SMILES | CC(=CCC1C2(CCCC(C2CCC1(C)O)(C)C)C)C=C | ||
| Standard InChIKey | ZAZVCYBIABTSJR-SZAPHMHZSA-N | ||
| Standard InChI | InChI=1S/C20H34O/c1-7-15(2)9-10-17-19(5)13-8-12-18(3,4)16(19)11-14-20(17,6)21/h7,9,16-17,21H,1,8,10-14H2,2-6H3/b15-9-/t16-,17+,19-,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

cis-Abienol Dilution Calculator

cis-Abienol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4423 mL | 17.2117 mL | 34.4234 mL | 68.8468 mL | 86.0585 mL |
| 5 mM | 0.6885 mL | 3.4423 mL | 6.8847 mL | 13.7694 mL | 17.2117 mL |
| 10 mM | 0.3442 mL | 1.7212 mL | 3.4423 mL | 6.8847 mL | 8.6059 mL |
| 50 mM | 0.0688 mL | 0.3442 mL | 0.6885 mL | 1.3769 mL | 1.7212 mL |
| 100 mM | 0.0344 mL | 0.1721 mL | 0.3442 mL | 0.6885 mL | 0.8606 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7-(3'-Carboxybutoxy)coumarin
Catalog No.:BCN9549
CAS No.:16851-01-7
- Buxifoliadine H
Catalog No.:BCN9548
CAS No.:263007-72-3
- Coryximine
Catalog No.:BCN9547
CAS No.:127460-61-1
- 6-Methoxykaempferol 3-O-galactoside
Catalog No.:BCN9546
CAS No.:72945-43-8
- Santin
Catalog No.:BCN9545
CAS No.:27782-63-4
- 5,5'-Dihydroxy-3,8,3',4'-tetramethoxy-6,7-methylenedioxyflavone
Catalog No.:BCN9544
CAS No.:82668-96-0
- 4"-O-Acetylastilbin
Catalog No.:BCN9543
CAS No.:1298135-49-5
- (1R,6R,9R)-6,9,11-Trihydroxy-4,7-megastigmadien-3-one 11-O-glucoside
Catalog No.:BCN9542
CAS No.:289914-68-7
- Atalafoline
Catalog No.:BCN9541
CAS No.:107259-49-4
- Atalaphylline
Catalog No.:BCN9540
CAS No.:28233-35-4
- 8,8''-Biskoenigine
Catalog No.:BCN9539
CAS No.:477890-82-7
- Buxifoliadine C
Catalog No.:BCN9538
CAS No.:263007-67-6
- Aesculioside D
Catalog No.:BCN9551
CAS No.:254896-66-7
- Ampelopsin A
Catalog No.:BCN9552
CAS No.:130608-11-6
- Kaempferol 3,5-dimethyl ether
Catalog No.:BCN9553
CAS No.:1486-65-3
- Acersaponin I
Catalog No.:BCN9554
CAS No.:1257940-29-6
- 2,3-Dihydro-6-methylginkgetin
Catalog No.:BCN9555
CAS No.:1013649-09-6
- 3-Formylcarbazole
Catalog No.:BCN9556
CAS No.:51761-07-0
- Vitisin A
Catalog No.:BCN9557
CAS No.:142449-89-6
- Vanicoside B
Catalog No.:BCN9558
CAS No.:155179-21-8
- Sonderianol
Catalog No.:BCN9559
CAS No.:85563-65-1
- Isoaesculioside D
Catalog No.:BCN9560
CAS No.:1184581-59-6
- Aesculioside C
Catalog No.:BCN9561
CAS No.:254896-65-6
- Quercetin 3,5,3'-trimethyl ether
Catalog No.:BCN9562
CAS No.:13459-09-1
Lipophilic Metabolites from Five-Needle Pines, Pinus armandii and Pinus kwangtungensis, Exhibiting Antibacterial Activity.[Pubmed:32413199]
Chem Biodivers. 2020 May 15.
Lipophilic extractive metabolites from needles and defoliated twigs of Pinus armandii and P. kwangtungensis were studied by GC/MS. Needles of P. armandii contained predominantly 15-O-functionalized labdane type acids (anticopalic acid), fatty acids, nonacosan-10-ol, sterols, nonacosan-10-ol and sterol saponifiable esters, and acylglycerols, while P. kwangtungensis needles contained no anticopalic acid, but more trinorlabdane (14,15,16-trinor-8(17)-labdene-13,19-dioic acid) and other labdane type acids, nonacosan-10-ol and its saponifiable esters. The major compounds in the P. armandii defoliated twig extract were abietane and isopimarane type acids, fatty acids, sterols, labdanoids (cis-Abienol), cembranoids (isocembrol and 4-epi-isocembrol), saponifiable sterol esters, and acylglycerols. The same extract of P. kwangtungensis contained larger quantities of fatty acids, caryophyllene oxide, serratanoids, sterols, saponifiable sterol esters, and acylglycerols, but lesser amounts of abietane and isopimarane type acids, cis-Abienol, and lacked cembranoids. Both twig and needle extracts of P. armandii and P. kwangtungensis, as well as the extracts' fractions, significantly inhibited the growth of Gram-negative bacteria Serratia marcescens with MIC of 0.1 mg ml(-1) , while in most cases they slightly stimulated the growth of Gram-positive bacteria Bacillus subtilis at the same concentrations. Thus, lipophilic extractive compounds from the needles and defoliated twigs of both pines are prospective for the development of antiseptics against Gram-negative bacteria.
Combinatorial Engineering of Mevalonate Pathway and Diterpenoid Synthases in Escherichia coli for cis-Abienol Production.[Pubmed:31117507]
J Agric Food Chem. 2019 Jun 12;67(23):6523-6531.
Identification of diterpene synthase-encoding genes together with synthetic biology technology offers an opportunity for the biosynthesis of cis-Abienol. The methylerythritol phosphate (MEP) and the mevalonate (MVA) pathways were both engineered for cis-Abienol production in Escherichia coli, which improved the cis-Abienol yield by approximately 7-fold and 31-fold, respectively, compared to the yield obtained by overexpression of the MEP pathway alone or the original MEP pathway. Furthermore, systematic optimization of cis-Abienol biosynthesis was performed, such as diterpene synthase screening and two-phase cultivation. The combination of bifunctional class I/II cis-Abienol synthase from Abies balsamea ( AbCAS) and class II abienol synthase from Salvia sclarea ( SsTPS2) was found to be the most effective. By using isopropyl myristate as a solvent in two-phase cultivation, cis-Abienol production reached 634.7 mg/L in a fed-batch bioreactor. This work shows the possibility of E. coli utilizing glucose as a carbon source for cis-Abienol biosynthesis through a modified pathway.
Pilot-scale cultivation of wall-deficient transgenic Chlamydomonas reinhardtii strains expressing recombinant proteins in the chloroplast.[Pubmed:26969037]
Appl Microbiol Biotechnol. 2016 Aug;100(16):7061-70.
Microalgae have emerged as potentially powerful platforms for the production of recombinant proteins and high-value products. Chlamydomonas reinhardtii is a potentially important host species due to the range of genetic tools that have been developed for this unicellular green alga. Transformation of the chloroplast genome offers important advantages over nuclear transformation, and a wide range of recombinant proteins have now been expressed in the chloroplasts of C. reinhardtii strains. This is often done in cell wall-deficient mutants that are easier to transform. However, only a single study has reported growth data for C. reinhardtii grown at pilot scale, and the growth of cell wall-deficient strains has not been reported at all. Here, we report the first pilot-scale growth study for transgenic, cell wall-deficient C. reinhardtii strains. Strains expressing a cytochrome P450 (CYP79A1) or bifunctional diterpene synthase (cis-Abienol synthase, TPS4) were grown for 7 days under mixotrophic conditions in a Tris-acetate-phosphate medium. The strains reached dry cell weights of 0.3 g/L within 3-4 days with stable expression levels of the recombinant proteins during the whole upscaling process. The strains proved to be generally robust, despite the cell wall-deficient phenotype, but grew poorly under phototrophic conditions. The data indicate that cell wall-deficient strains may be highly amenable for transformation and suitable for commercial-scale operations under mixotrophic growth regimes.
Enzymes for synthetic biology of ambroxide-related diterpenoid fragrance compounds.[Pubmed:25846965]
Adv Biochem Eng Biotechnol. 2015;148:427-47.
Ambrox and related ambroxides are highly priced in the fragrance industry, and valued for their delicate odor and fixative properties. Historically, ambrox was obtained from ambergris, a waxy excretion produced by sperm whales, now an endangered species. Synthetic ambroxides have replaced ambergris in perfume manufacture. Plant labdane diterpenoids can serve as starting material for ambroxide synthesis. Among these, the diterpene alcohol sclareol is the major industrial precursor obtained from cultivated clary sage (Salvia sclarea). In plants, a large family of diterpene synthase (diTPS) enzymes controls key reactions in diterpenoid biosynthesis. Advanced metabolite profiling and high-throughput sequencing of fragrant and medicinal plants have accelerated discovery of novel diTPS functions, providing a resource for combinatorial synthetic biology and metabolic engineering approaches. This chapter highlights recent progress on the discovery, characterization, and engineering of plant diTPSs with potential uses in ambroxide production. It features biosynthesis of sclareol, cis-Abienol, and diterpene resin acids, as sources of genes and enzymes for diterpenoid bioproducts.
Efficient diterpene production in yeast by engineering Erg20p into a geranylgeranyl diphosphate synthase.[Pubmed:25446975]
Metab Eng. 2015 Jan;27:65-75.
Terpenes have numerous applications, ranging from pharmaceuticals to fragrances and biofuels. With increasing interest in producing terpenes sustainably and economically, there has been significant progress in recent years in developing methods for their production in microorganisms. In Saccharomyces cerevisiae, production of the 20-carbon diterpenes has so far proven to be significantly less efficient than production of their 15-carbon sesquiterpene counterparts. In this report, we identify the modular structure of geranylgeranyl diphosphate synthesis in yeast to be a major limitation in diterpene yields, and we engineer the yeast farnesyl diphosphate synthase Erg20p to produce geranylgeranyl diphosphate. Using a combination of protein and genetic engineering, we achieve significant improvements in the production of sclareol and several other isoprenoids, including cis-Abienol, abietadiene and beta-carotene. We also report the development of yeast strains carrying the engineered Erg20p, which support efficient isoprenoid production and can be used as a dedicated chassis for diterpene production or biosynthetic pathway elucidation. The design developed here can be applied to the production of any GGPP-derived isoprenoid and is compatible with other yeast terpene production platforms.
Identification of natural diterpenes that inhibit bacterial wilt disease in tobacco, tomato and Arabidopsis.[Pubmed:22685082]
Plant Cell Physiol. 2012 Aug;53(8):1432-44.
The soil-borne bacterial pathogen Ralstonia solanacearum invades a broad range of plants through their roots, resulting in wilting of the plant, but no effective protection against this disease has been developed. Two bacterial wilt disease-inhibiting compounds were biochemically isolated from tobacco and identified as sclareol and cis-Abienol, labdane-type diterpenes. When exogenously applied to their roots, sclareol and cis-Abienol inhibited wilt disease in tobacco, tomato and Arabidopsis plants without exhibiting any antibacterial activity. Microarray analysis identified many sclareol-responsive genes in Arabidopsis roots, including genes encoding or with a role in ATP-binding cassette (ABC) transporters, and biosynthesis and signaling of defense-related molecules and mitogen-activated protein kinase (MAPK) cascade components. Inhibition of wilt disease by sclareol was attenuated in Arabidopsis mutants defective in the ABC transporter AtPDR12, the MAPK MPK3, and ethylene and abscisic acid signaling pathways, and also in transgenic tobacco plants with reduced expression of NtPDR1, a tobacco homolog of AtPDR12. These results suggest that multiple host factors are involved in the inhibition of bacterial wilt disease by sclareol-related compounds.
Bifunctional cis-abienol synthase from Abies balsamea discovered by transcriptome sequencing and its implications for diterpenoid fragrance production.[Pubmed:22337889]
J Biol Chem. 2012 Apr 6;287(15):12121-31.
The labdanoid diterpene alcohol cis-Abienol is a major component of the aromatic oleoresin of balsam fir (Abies balsamea) and serves as a valuable bioproduct material for the fragrance industry. Using high-throughput 454 transcriptome sequencing and metabolite profiling of balsam fir bark tissue, we identified candidate diterpene synthase sequences for full-length cDNA cloning and functional characterization. We discovered a bifunctional class I/II cis-Abienol synthase (AbCAS), along with the paralogous levopimaradiene/abietadiene synthase and isopimaradiene synthase, all of which are members of the gymnosperm-specific TPS-d subfamily. The AbCAS-catalyzed formation of cis-Abienol proceeds via cyclization and hydroxylation at carbon C-8 of a postulated carbocation intermediate in the class II active site, followed by cleavage of the diphosphate group and termination of the reaction sequence without further cyclization in the class I active site. This reaction mechanism is distinct from that of synthases of the isopimaradiene- or levopimaradiene/abietadiene synthase type, which employ deprotonation reactions in the class II active site and secondary cyclizations in the class I active site, leading to tricyclic diterpenes. Comparative homology modeling suggested the active site residues Asp-348, Leu-617, Phe-696, and Gly-723 as potentially important for the specificity of AbCAS. As a class I/II bifunctional enzyme, AbCAS is a promising target for metabolic engineering of cis-Abienol production.
Phytochemicals from Cunninghamia konishii Hayata act as antifungal agents.[Pubmed:22129092]
J Agric Food Chem. 2012 Jan 11;60(1):124-8.
The aims of the present study were to isolate and identify the antifungal compounds from the ethanolic extract of Cunninghamia konishii wood and to evaluate their antifungal activities against wood decay fungi. The results showed that the n-Hex soluble fraction of the ethanolic extract from C. konishii wood had an excellent inhibitory effect against Lenzites betulina, Trametes versicolor, Laetiporus sulphureus, and Gloeophyllum trabeum, with IC(50) values of 33, 46, 62, and 49 mug/mL, respectively. By following the bioactivity-guided fractionation procedure, four sesquiterpenes, T-cadinol, cedrol, T-muurolol, and (-)-epi-cedrol, and three diterpenes, 13-epi-manool, cis-Abienol, and isoabienol, were identified from the active subfractions. Among the main constituents of the ethanolic extract from C. konishii, T-cadinol, cedrol, and T-muurolol efficiently inhibited the growth of four wood-rot fungi at the concentration of 100 mug/mL, with antifungal indices of 51.4-100.0%, 68.3-100.0%, and 39.5-100.0%, respectively. Results of this study show that the ethanolic extract of C. konishii wood may be considered as a potent source of T-cadinol, cedrol, and T-muurolol as new natural antifungal agents.
Combined analysis by GC (RI), GC-MS and 13C NMR of the supercritical fluid extract of Abies alba twigs.[Pubmed:21299139]
Nat Prod Commun. 2010 Dec;5(12):1995-8.
Two samples (leaves and twigs) of Abies alba Miller from Corsica were extracted using supercritical CO2 and their chemical compositions were compared with those of the essential oils obtained from the same batch of plant material. In total 45 components were identified using combined analysis by GC (RI), GC-MS and 13C NMR. It was observed that the contents of monoterpenes (mainly represented by limonene, alpha-pinene and camphene) were significantly lower in the supercritical fluid extract (SFE) than in the essential oil (EO). Conversely, the proportions of sesquiterpenes were much higher in CO2 extracts than in essential oils (around 30% vs 4%). cis-Abienol, a diterpene alcohol, was identified only in SFE, and the proportions of this constituent (7.5% and 17.3%) were determined using quantitative 13C NMR since it was under estimated using the standard conditions of GC.


