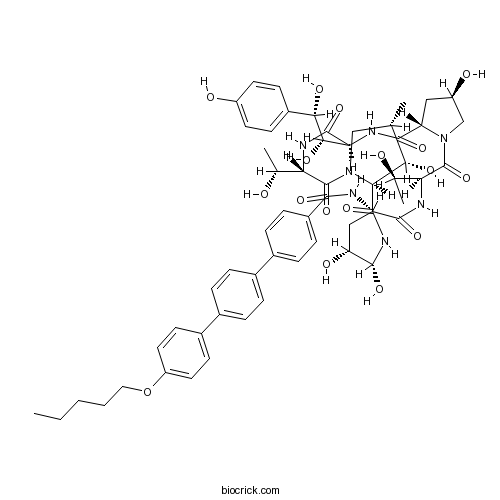
AnidulafunginAntifungal drug CAS# 166663-25-8 |
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Tinidazole
Catalog No.:BCC4866
CAS No.:19387-91-8
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 166663-25-8 | SDF | Download SDF |
| PubChem ID | 166548 | Appearance | Powder |
| Formula | C58H73N7O17 | M.Wt | 1140.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | LY303366 | ||
| Solubility | DMSO : ≥ 30 mg/mL (26.31 mM) *"≥" means soluble, but saturation unknown. | ||
| SMILES | CCCCCOC1=CC=C(C=C1)C2=CC=C(C=C2)C3=CC=C(C=C3)C(=O)NC4CC(C(NC(=O)C5C(C(CN5C(=O)C(NC(=O)C(NC(=O)C6CC(CN6C(=O)C(NC4=O)C(C)O)O)C(C(C7=CC=C(C=C7)O)O)O)C(C)O)C)O)O)O | ||
| Standard InChIKey | JHVAMHSQVVQIOT-MFAJLEFUSA-N | ||
| Standard InChI | InChI=1S/C58H73N7O17/c1-5-6-7-24-82-40-22-18-35(19-23-40)33-10-8-32(9-11-33)34-12-14-37(15-13-34)51(74)59-41-26-43(70)54(77)63-56(79)47-48(71)29(2)27-65(47)58(81)45(31(4)67)61-55(78)46(50(73)49(72)36-16-20-38(68)21-17-36)62-53(76)42-25-39(69)28-64(42)57(80)44(30(3)66)60-52(41)75/h8-23,29-31,39,41-50,54,66-73,77H,5-7,24-28H2,1-4H3,(H,59,74)(H,60,75)(H,61,78)(H,62,76)(H,63,79)/t29-,30+,31+,39+,41-,42-,43+,44-,45-,46-,47-,48-,49-,50-,54+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Anidulafungin is a new semisynthetic echinocandin with antifungal potency.In Vitro:Anidulafungin (LY-303366) has MICs of ≤0.32 μg/mL for all Candida albicans (n=99), Candida glabrata (n=18), and Candida tropicalis (n=10) isolates tested. Anidulafungin is also active against Aspergillus species (minimum effective concentration at which 90% of the isolates are inhibited, 0.02 μg/mL) (n=20), is less active against Candida parapsilosis (MIC at which 90% of the isolates are inhibited [MIC90], 5.12 μg/mL) (n=10), and is inactive against C. neoformans (MIC90 >10.24 μg/mL) (n=15) and B. dermatitidis (MIC90, 16 μg/mL) (n=29).The MICs of Fluconazole for three strains of C. tropicalis, seven strains of C. glabrata, and two strains of Candida krusei are ≥16 μg/mL. The MICs of Anidulafungin for 11 of these 12 strains range from 0.08 to 0.32 mg/mL. The twelfth strain is a C. krusei strain (Fluconazole MIC, 32 μg/mL) for which the Anidulafungin MIC is 1.28 mg/mL. The MIC at which 90% of the isolates are inhibited (MIC90) for these 12 strains is 0.32 μg/mL. The Anidulafungin MIC90 for the remaining 18 C. glabrata isolates and C. tropicalis isolates for which the Fluconazole MICs are ≥ 8 μg/mL is also 0.32 mg/mL. Anidulafungin appeares equally active against Candida species for which the fluconazole MICs are ≥16 mg/mL and against those for which the fluconazole MICs are ≥ 8 μg/mL. Anidulafungin has significantly less activity against C. neoformans and B. dermatitidis than against C. albicans, C. glabrata, and C. tropicalis. Ketoconazole and amphotericin B are the most active antifungal agents tested for both C. neoformans and B. dermatitidis. Anidulafungin demonstrated potent in vitro activity against Aspergillus species with a MEC90 of 0.02 μg/mL. MICs of Anidulafungin for the control strain yeast isolates are 0.02 μg/mL for C. albicans ATCC 90028, 0.16 mg/mL for C. glabrata ATCC 90030, and >10.24 μg/mL for C. neoformans ATCC 90112[1]. Strains selected with CD101 that have a 2-fold or greater CD101 MIC increase also have at least a 2-fold MIC increase for Anidulafungin (ANF) and/or Caspofungin (CSF)[2]. References: | |||||

Anidulafungin Dilution Calculator

Anidulafungin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.877 mL | 4.385 mL | 8.7701 mL | 17.5402 mL | 21.9252 mL |
| 5 mM | 0.1754 mL | 0.877 mL | 1.754 mL | 3.508 mL | 4.385 mL |
| 10 mM | 0.0877 mL | 0.4385 mL | 0.877 mL | 1.754 mL | 2.1925 mL |
| 50 mM | 0.0175 mL | 0.0877 mL | 0.1754 mL | 0.3508 mL | 0.4385 mL |
| 100 mM | 0.0088 mL | 0.0439 mL | 0.0877 mL | 0.1754 mL | 0.2193 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Anidulafungin (LY-303366)is a synthetic derivative of echinocandin B, used as a drug of antifungal [1].
Anidulafungin has been reported to inhibit systemic isolates of Candida species with the MIC90 values of 0.08μg/ml, 0.32μg/ml, 0.32μg/ml and 5.12μg/ml for C.albicans (n=99), C.glabrata(n=18), C.tropicalis(n=10), and C.parapsilosis (n=10), respectively. In addition, Anidulafungin has been found to be active against Aspergillus species(n=20) with the MIC90 values of ~0.02μg/ml. Apart from these, Anidulafungin has been revealed to inactively restrain with the MIC90 values of >10.24μg/ml and 16μg/ml, for C.neoformans and B.dermatitidis, respectively [1].
References:
[1] Zhanel GG1, Karlowsky JA, Harding GA, Balko TV, Zelenitsky SA, Friesen M, Kabani A, Turik M, Hoban DJ. In vitro activity of a new semisynthetic echinocandin, LY-303366, against systemic isolates of Candida species, Cryptococcus neoformans, Blastomyces dermatitidis, and Aspergillus species. Antimicrob Agents Chemother. 1997 Apr;41(4):863-5.
- Ramelteon
Catalog No.:BCN2183
CAS No.:196597-26-9
- Eurycarpin A
Catalog No.:BCN4693
CAS No.:166547-20-2
- H-Val-OBzl.TosOH
Catalog No.:BCC3140
CAS No.:16652-76-9
- H-Pro-OBzl.HCl
Catalog No.:BCC3021
CAS No.:16652-71-4
- H-Tyr(Bzl)-OH
Catalog No.:BCC3130
CAS No.:16652-64-5
- Avasimibe
Catalog No.:BCC2274
CAS No.:166518-60-1
- Metaxalone
Catalog No.:BCC5223
CAS No.:1665-48-1
- trans-4-(Trifluoromethyl)cinnamic acid
Catalog No.:BCN1534
CAS No.:16642-92-5
- p-Nitrohydrocinnamic acid
Catalog No.:BCC9124
CAS No.:16642-79-8
- 1H-Pyrido[3,4-b]indole-1,3 4(2H,9H)-trione
Catalog No.:BCN7913
CAS No.:16641-79-5
- m-Nitrohydrocinnamic acid
Catalog No.:BCC9048
CAS No.:1664-57-9
- 9-O-Feruloyl-5,5'-dimethoxylariciresinol
Catalog No.:BCN1535
CAS No.:166322-14-1
- 4,4'-Bis(chloromethyl)biphenyl
Catalog No.:BCC8658
CAS No.:1667-10-3
- cis-Mulberroside A
Catalog No.:BCN3911
CAS No.:166734-06-1
- H-Cys(Trt)-NH2
Catalog No.:BCC2912
CAS No.:166737-85-5
- Naltrexone HCl
Catalog No.:BCC4613
CAS No.:16676-29-2
- Z-Tyr(Bzl)-OH
Catalog No.:BCC2735
CAS No.:16677-29-5
- Prerubialatin
Catalog No.:BCN6895
CAS No.:1667718-89-9
- Desmopressin
Catalog No.:BCC1525
CAS No.:16679-58-6
- H-Gly-NH2.HCl
Catalog No.:BCC2947
CAS No.:1668-10-6
- H-D-Orn-OH. HCl
Catalog No.:BCC3004
CAS No.:16682-12-5
- Chebulanin
Catalog No.:BCN3261
CAS No.:166833-80-3
- A-1210477
Catalog No.:BCC6508
CAS No.:1668553-26-1
- 3-Amino-5-mercapto-1,2,4-triazole
Catalog No.:BCC8615
CAS No.:16691-43-3
Cost-effectiveness analysis of combination antifungal therapy with voriconazole and anidulafungin versus voriconazole monotherapy for primary treatment of invasive aspergillosis in Spain.[Pubmed:28115858]
Clinicoecon Outcomes Res. 2016 Dec 30;9:39-47.
OBJECTIVE: According to a recent randomized, double-blind clinical trial comparing the combination of voriconazole and Anidulafungin (VOR+ANI) with VOR monotherapy for invasive aspergillosis (IA) in patients with hematologic disease or with hematopoietic stem cell transplant, mortality was lower after 6 weeks with VOR+ANI than with VOR monotherapy in a post hoc analysis of patients with galactomannan-based IA. The objective of this study was to compare the cost-effectiveness of VOR+ANI with VOR, from the perspective of hospitals in the Spanish National Health System. METHODS: An economic model with deterministic and probabilistic analyses was used to determine costs per life-year gained (LYG) for VOR+ANI versus VOR in patients with galactomannan-based IA. Mortality, adverse event rates, and life expectancy were obtained from clinical trial data. The costs (in 2015 euros [euro]) of the drugs and the adverse event-related costs were obtained from Spanish sources. A Tornado plot and a Monte Carlo simulation (1,000 iterations) were used to assess uncertainty of all model variables. RESULTS: According to the deterministic analysis, for each patient treated with VOR+ANI compared with VOR monotherapy, there would be a total of 0.348 LYG (2.529 vs 2.181 years, respectively) at an incremental cost of euro5,493 (euro17,902 vs euro12,409, respectively). Consequently, the additional cost per LYG with VOR+ANI compared with VOR would be euro15,785. Deterministic sensitivity analyses confirmed the robustness of these findings. In the probabilistic analysis, the cost per LYG with VOR+ANI was euro15,774 (95% confidence interval: euro15,763-16,692). The probability of VOR+ANI being cost-effective compared with VOR was estimated at 82.5% and 91.9%, based on local cost-effectiveness thresholds of euro30,000 and euro45,000, respectively. CONCLUSION: According to the present economic study, combination therapy with VOR+ANI is cost-effective as primary therapy of IA in galactomannan-positive patients in Spain who have hematologic disease or hematopoietic stem cell transplant, compared with VOR monotherapy.
Pharmacokinetic study of anidulafungin in ICU patients with intra-abdominal candidiasis.[Pubmed:28088767]
J Antimicrob Chemother. 2017 May 1;72(5):1429-1432.
Background: Only limited pharmacokinetic data are available for Anidulafungin in ICU patients, especially in patients treated for severe intra-abdominal infection (IAI). Methods: This was a prospective multicentre observational study in ICU patients with suspected yeast IAI. All patients received an intravenous loading dose of 200 mg of Anidulafungin, followed by 100 mg/day. Thirteen blood samples were drawn between day 1 and day 5 for pharmacokinetic analysis. Samples were analysed by an HPLC-tandem MS method. Demographics and SAPS2 and SOFA scores were recorded. Results: Fourteen patients with a median age (IQR) of 62 years (48-70) and with a mean BMI of 30.5 kg/m 2 were included from three centres; 57.1% were women. Their median (IQR) SAPS2 score was 54 (45-67) and their median (IQR) SOFA score was 8 (7-12). Six patients with community-acquired IAI and eight patients with nosocomial-acquired IAI were included. Twelve yeasts were isolated: six Candida albicans , two Candida glabrata , two Candida tropicalis , one Candida parapsilosis and one Candida krusei . Pharmacokinetic parameters were as follows [mean (% coefficient of variation)]: C max (mg/L) = 6.0 (29%); T max (h) = 1.6 (25.8%); C min (mg/L) = 3.2 (36.8%); AUC 0-24 (mg.h/L) = 88.9 (38.6%); t 1/2 (h) = 42.1 (68.2%); CL (L/h) = 1.2 (42.3%); and V (L) = 72.8 (87.8%). A two-compartment model best described the Anidulafungin concentrations in the population pharmacokinetic study. Conclusions: The pharmacokinetic parameters of Anidulafungin in critically ill ICU patients with complicated IAI are similar to those observed in the literature. However, an increased V and a longer t 1/2 were observed in this study. (EudraCT No. 2010-018695-25).
In Vitro Activity of Posaconazole against Talaromyces marneffei by Broth Microdilution and Etest Methods and Comparison to Itraconazole, Voriconazole, and Anidulafungin.[Pubmed:28031205]
Antimicrob Agents Chemother. 2017 Feb 23;61(3). pii: AAC.01480-16.
We determined the susceptibilities of 57 Talaromyces marneffei strains to Anidulafungin, itraconazole, voriconazole, and posaconazole with MICs of 2 to 8, 0.002 to 0.004, 0.016 to 0.063, and 0.001 to 0.002 mug/ml by broth microdilution and >32, Anidulafungin and posaconazole were 1 to 2 and 0.004 to 0.063 mug/ml, respectively. The results suggest promising activities of posaconazole. Etest can be used for testing of azoles against T. marneffei.
Pharmacokinetics of Anidulafungin in Critically Ill Intensive Care Unit Patients with Suspected or Proven Invasive Fungal Infections.[Pubmed:27872072]
Antimicrob Agents Chemother. 2017 Jan 24;61(2). pii: AAC.01894-16.
Echinocandins, such as Anidulafungin, are the first-line treatment for candidemia or invasive candidiasis in critically ill patients. There are conflicting data on the pharmacokinetic properties of Anidulafungin in intensive care unit (ICU) patients. Adult ICU patients (from 3 hospitals) receiving Anidulafungin for suspected or proven fungal infections were included in the present study. Patients were considered evaluable if a pharmacokinetic curve for day 3 could be completed. Twenty-three of 36 patients (7 female and 16 male) were evaluable. The median (range) age and body weight were 66 (28 to 88) years and 76 (50 to 115) kg, respectively. Pharmacokinetic sampling on day 3 (n = 23) resulted in a median Anidulafungin area under the concentration-time curve from 0 to 24 h (AUC0-24) of 72.1 (interquartile range [IQR], 61.3 to 94.0) mg . h . liter(-1), a median daily trough concentration (C24) of 2.2 (IQR, 1.9 to 2.9) mg/liter, a median maximum concentration of drug in serum (Cmax) of 5.3 (IQR, 4.1 to 6.0) mg/liter, a median volume of distribution (V) of 46.0 (IQR, 32.2 to 60.2) liters, and a median clearance (CL) of 1.4 (IQR, 1.1 to 1.6) liters . h(-1) Pharmacokinetic sampling on day 7 (n = 13) resulted in a median AUC0-24 of 82.7 (IQR, 73.0 to 129.5) mg . h . liter(-1), a median minimum concentration of drug in serum (Cmin) of 2.8 (IQR, 2.2 to 4.2) mg/liter, a median Cmax of 5.9 (IQR, 4.6 to 8.0) mg/liter, a median V of 39.7 (IQR, 32.2 to 54.4) liters, and a median CL of 1.2 (IQR, 0.8 to 1.4) liters . h(-1) The geometric mean ratio for the AUCday7/AUCday3 term was 1.13 (90% confidence interval [CI], 1.03 to 1.25). The exposure in the ICU patient population was in accordance with previous reports on Anidulafungin pharmacokinetics in ICU patients but was lower than that for healthy volunteers or other patient populations. Larger cohorts of patients or pooled data analyses are necessary to retrieve relevant covariates. (This study has been registered at ClinicalTrials.gov under identifier NCT01438216.).


