trans-4-(Trifluoromethyl)cinnamic acidCAS# 16642-92-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 16642-92-5 | SDF | Download SDF |
| PubChem ID | 5553364 | Appearance | White cryst. |
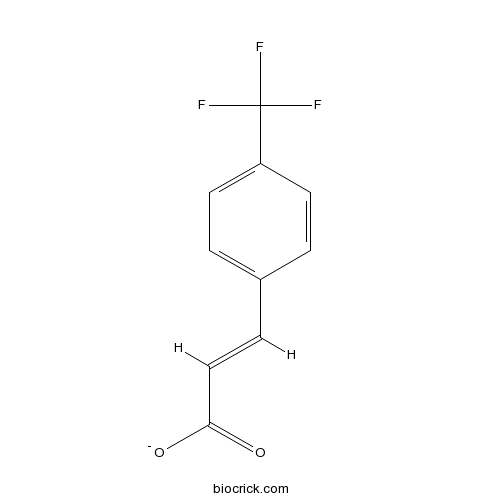
| Formula | C10H7F3O2 | M.Wt | 216.16 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-3-[4-(trifluoromethyl)phenyl]prop-2-enoate | ||
| SMILES | C1=CC(=CC=C1C=CC(=O)[O-])C(F)(F)F | ||
| Standard InChIKey | ANRMAUMHJREENI-ZZXKWVIFSA-M | ||
| Standard InChI | InChI=1S/C10H7F3O2/c11-10(12,13)8-4-1-7(2-5-8)3-6-9(14)15/h1-6H,(H,14,15)/p-1/b6-3+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | trans-4-(Trifluoromethyl)cinnamic acid is a photosensitive compound. |
| In vitro | Photosensitive semiconductor nanocrystals, photosensitive composition comprising semiconductor nanocrystals and method for forming semiconductor nanocrystal pattern using the same[Reference: WebLink]US 8758864 B2[P]. 2014.4. The organic-inorganic hybrid electroluminescent device according to claim 1, wherein the compound containing a photosensitive functional group is selected from a group consisting of methacrylic acid, crotonic acid, vinylacetic acid, tiglic acid, 3,3-dimethylacrylic acid, trans-2-pentenoic acid, 4-pentenoic acid, trans-2-methyl-2-pentenoic acid, 2,2-dimethyl-4-pentenoic acid, trans-2-hexenoic acid, trans-3-hexenoic acid, 2-ethyl-2-hexenoic acid, 6-heptenoic acid, 2-octenoic acid, citronellic acid, undecylenic acid, myristoleic acid, palmitoleic acid, oleic acid, elaidic acid, cis-11-elcosenoic acid, euric acid, nervonic acid, trans-2,4-pentadienoic acid, 2,4-hexadienoic acid, 2,6-heptadienoic acid, geranic acid, linoleic acid, 11,14-eicosadienoic acid, cis-8,11,14-eicosatrienoic acid, arachidonic acid, cis-5,8,11,14,17-eicosapentaenoic acid, cis-4,7,10,13,16,19-docosahexaenoic acid, fumaric acid, maleic acid, itaconic acid, ciraconic acid, mesaconic acid, trans-glutaconic acid, trans-beta-hydromuconic acid, trans-traumatic acid, trans-muconic acid, cis-aconitic acid, trans-aconitic acid, cis-3-chloroacrylic acid, trans-3-chloroacrylic acid, 2-bromoacrylic acid, 2-(trifluoromethyl)acryl-ic acid, trans-styrylacetic acid, trans-cinnamic acid, alpha.-methylcinnamic acid, 2-methylcinnamic acid, 2-fluorocinnamic acid, 2-(trifluoromethyl)cinnamic acid, 2-chlorocinnamic acid, 2-methoxycinnamic acid, 2-hydroxycinnamic acid, 2-nitrocinnamic acid, 2-carboxycinnamic acid, trans-3-fluorocinnamic acid, 3-(trifluoromethyl)cinnamic acid, 3-chlorocinnamic acid, 3-bromocinnamic acid, 3-methoxycinnamic acid, 3-hydroxycinnamic acid, 3-nitrocinnamic acid, 4-methylcinnamic acid, 4-fluorocinnamic acid, trans-4-(Trifluoromethyl)cinnamic acid, 4-chlorocinnamic acid, 4-bromocinnamic acid, 4-methoxycinnamic acid, 4-hydroxycinnamic acid, 4-nitrocinnamic acid, 3,3-dimethoxycinnamic acid, 4-vinylbenzoic acid, allyl methyl sulfide, allyl disulfide, diallyl amine, oleylamine, 3-amino-1-propanol vinyl ether, 4-chlorocinnamonitrile, 4-methoxycinnamonitrile, 3,4-dimethoxycinnamonitrile, 4-dimethylaminocinnamonitrile, acrylonitrile, allyl cyanide, crotononitrile, methacrylonitrile, cis-2-pentenenitrile, trans-3-pentenenitrile, 3,7-dimethyl-2,6-octadienenitrile, and 1,4-dicyano-2-butene. |
| Structure Identification | CrystEngComm.2008 Jan 10;2(5):502-506.Trans-4-(trifluoromethyl) cinnamic acid: Structural characterisation and crystallographic investigation into the temperature induced phase transition.[Reference: WebLink]The crystal structure of trans-4-(Trifluoromethyl)cinnamic acid (1) has been determined in the triclinic space groupP[1 with combining macron]. Differential scanning calorimetry showed that trans-4-(Trifluoromethyl)cinnamic acid undergoes a single fully reversible temperature induced phase transition at around 132/131 K (cooling/heating). The structures are stabilised by the presence of O–H⋯O hydrogen bonding and C–H⋯O interactions. The results of DSC, single crystal and powder X-ray diffraction studies on trans-4-(Trifluoromethyl)cinnamic acid are reported. |

trans-4-(Trifluoromethyl)cinnamic acid Dilution Calculator

trans-4-(Trifluoromethyl)cinnamic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.6262 mL | 23.131 mL | 46.262 mL | 92.5241 mL | 115.6551 mL |
| 5 mM | 0.9252 mL | 4.6262 mL | 9.2524 mL | 18.5048 mL | 23.131 mL |
| 10 mM | 0.4626 mL | 2.3131 mL | 4.6262 mL | 9.2524 mL | 11.5655 mL |
| 50 mM | 0.0925 mL | 0.4626 mL | 0.9252 mL | 1.8505 mL | 2.3131 mL |
| 100 mM | 0.0463 mL | 0.2313 mL | 0.4626 mL | 0.9252 mL | 1.1566 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- p-Nitrohydrocinnamic acid
Catalog No.:BCC9124
CAS No.:16642-79-8
- 1H-Pyrido[3,4-b]indole-1,3 4(2H,9H)-trione
Catalog No.:BCN7913
CAS No.:16641-79-5
- m-Nitrohydrocinnamic acid
Catalog No.:BCC9048
CAS No.:1664-57-9
- 9-O-Feruloyl-5,5'-dimethoxylariciresinol
Catalog No.:BCN1535
CAS No.:166322-14-1
- Taberdivarine H
Catalog No.:BCN6958
CAS No.:1662688-34-7
- Argentinine
Catalog No.:BCN3987
CAS No.:16625-57-3
- Bathophenanthroline
Catalog No.:BCC8840
CAS No.:1662-01-7
- CCT251545
Catalog No.:BCC6487
CAS No.:1661839-45-7
- Ginkgolic acid C15:0
Catalog No.:BCN2483
CAS No.:16611-84-0
- 3,4-O-dimethylcedrusin
Catalog No.:BCN8211
CAS No.:166021-14-3
- Cyclogrifolin
Catalog No.:BCN7547
CAS No.:1660156-04-6
- Z-Thr-OBzl
Catalog No.:BCC2737
CAS No.:16597-50-5
- Metaxalone
Catalog No.:BCC5223
CAS No.:1665-48-1
- Avasimibe
Catalog No.:BCC2274
CAS No.:166518-60-1
- H-Tyr(Bzl)-OH
Catalog No.:BCC3130
CAS No.:16652-64-5
- H-Pro-OBzl.HCl
Catalog No.:BCC3021
CAS No.:16652-71-4
- H-Val-OBzl.TosOH
Catalog No.:BCC3140
CAS No.:16652-76-9
- Eurycarpin A
Catalog No.:BCN4693
CAS No.:166547-20-2
- Ramelteon
Catalog No.:BCN2183
CAS No.:196597-26-9
- Anidulafungin
Catalog No.:BCC4233
CAS No.:166663-25-8
- 4,4'-Bis(chloromethyl)biphenyl
Catalog No.:BCC8658
CAS No.:1667-10-3
- cis-Mulberroside A
Catalog No.:BCN3911
CAS No.:166734-06-1
- H-Cys(Trt)-NH2
Catalog No.:BCC2912
CAS No.:166737-85-5
- Naltrexone HCl
Catalog No.:BCC4613
CAS No.:16676-29-2


