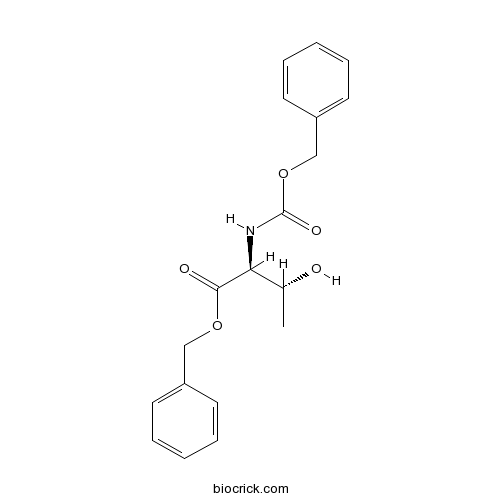
Z-Thr-OBzlCAS# 16597-50-5 |
- Dihydroberberine
Catalog No.:BCN2573
CAS No.:483-15-8
- Sesamolin
Catalog No.:BCN1289
CAS No.:526-07-8
- Carnosol
Catalog No.:BCN1055
CAS No.:5957-80-2
- Harpagide
Catalog No.:BCN4996
CAS No.:6926-08-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 16597-50-5 | SDF | Download SDF |
| PubChem ID | 7018808 | Appearance | Powder |
| Formula | C19H21NO5 | M.Wt | 343.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | benzyl (2S,3R)-3-hydroxy-2-(phenylmethoxycarbonylamino)butanoate | ||
| SMILES | CC(C(C(=O)OCC1=CC=CC=C1)NC(=O)OCC2=CC=CC=C2)O | ||
| Standard InChIKey | VBKUVUJWFDXTMS-PBHICJAKSA-N | ||
| Standard InChI | InChI=1S/C19H21NO5/c1-14(21)17(18(22)24-12-15-8-4-2-5-9-15)20-19(23)25-13-16-10-6-3-7-11-16/h2-11,14,17,21H,12-13H2,1H3,(H,20,23)/t14-,17+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Z-Thr-OBzl Dilution Calculator

Z-Thr-OBzl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9121 mL | 14.5603 mL | 29.1206 mL | 58.2411 mL | 72.8014 mL |
| 5 mM | 0.5824 mL | 2.9121 mL | 5.8241 mL | 11.6482 mL | 14.5603 mL |
| 10 mM | 0.2912 mL | 1.456 mL | 2.9121 mL | 5.8241 mL | 7.2801 mL |
| 50 mM | 0.0582 mL | 0.2912 mL | 0.5824 mL | 1.1648 mL | 1.456 mL |
| 100 mM | 0.0291 mL | 0.1456 mL | 0.2912 mL | 0.5824 mL | 0.728 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Z-Thr-OBzl
- (-)-Tetramisole
Catalog No.:BCC4670
CAS No.:16595-80-5
- Calystegine A5
Catalog No.:BCN1887
CAS No.:165905-26-0
- Naltrexone
Catalog No.:BCC1783
CAS No.:16590-41-3
- 1-Hydroxy-N-methylacridone
Catalog No.:BCN7551
CAS No.:16584-54-6
- Eperezolid
Catalog No.:BCC5177
CAS No.:165800-04-4
- Linezolid
Catalog No.:BCC2496
CAS No.:165800-03-3
- Torososide A
Catalog No.:BCN4694
CAS No.:165689-32-7
- Methyl 6-acetoxyangolensate
Catalog No.:BCN1732
CAS No.:16566-88-4
- L-Stepholidine
Catalog No.:BCN2599
CAS No.:16562-13-3
- 12-O-tetradecanoylphorbol-13-acetate
Catalog No.:BCN2511
CAS No.:16561-29-8
- DEPBT
Catalog No.:BCC2811
CAS No.:165534-43-0
- Di-Dnp-L-Lysine
Catalog No.:BCC8939
CAS No.:1655-49-8
- Cyclogrifolin
Catalog No.:BCN7547
CAS No.:1660156-04-6
- 3,4-O-dimethylcedrusin
Catalog No.:BCN8211
CAS No.:166021-14-3
- Ginkgolic acid C15:0
Catalog No.:BCN2483
CAS No.:16611-84-0
- CCT251545
Catalog No.:BCC6487
CAS No.:1661839-45-7
- Bathophenanthroline
Catalog No.:BCC8840
CAS No.:1662-01-7
- Argentinine
Catalog No.:BCN3987
CAS No.:16625-57-3
- Taberdivarine H
Catalog No.:BCN6958
CAS No.:1662688-34-7
- 9-O-Feruloyl-5,5'-dimethoxylariciresinol
Catalog No.:BCN1535
CAS No.:166322-14-1
- m-Nitrohydrocinnamic acid
Catalog No.:BCC9048
CAS No.:1664-57-9
- 1H-Pyrido[3,4-b]indole-1,3 4(2H,9H)-trione
Catalog No.:BCN7913
CAS No.:16641-79-5
- p-Nitrohydrocinnamic acid
Catalog No.:BCC9124
CAS No.:16642-79-8
- trans-4-(Trifluoromethyl)cinnamic acid
Catalog No.:BCN1534
CAS No.:16642-92-5
Synthesis of O-glycosylated tuftsins by utilizing threonine derivatives containing an unprotected monosaccharide moiety.[Pubmed:2119354]
Int J Pept Protein Res. 1990 Jul;36(1):86-96.
Synthesis is described of four tuftsin derivatives containing a D-glucopyranosyl or a D-galactopyranosyl unit covalently linked to the hydroxy side chain function of the threonine residue through either an alpha or beta O-glycosidic linkage. Fmoc-threonine derivatives containing the suitable unprotected sugar were used for incorporating the O-glycosylated amino acid residue. Z-Thr[alpha-Glc(OBzl)4]-OBzl and Z-Thr[alpha-Gal(OBzl)4]-OBzl were prepared from the tetra-O-benzylated sugar and Z-Thr-OBzl by the trichloroacetimidate method in the presence of trimethylsilyl trifluoromethane sulfonate. The alpha glycosylated threonine derivatives were converted into Fmoc-Thr(alpha-Glc)-OH and Fmoc-Thr(alpha-Gal)-OH by catalytic hydrogenation followed by acylation with Fmoc-OSu. beta-Glucosylation and beta-galactosylation of threonine were carried out by reacting the proper per-O-acetylated sugar with Z-Thr-OBzl and boron trifluoride ethyl etherate in dichloromethane. Catalytic hydrogenation of the beta-O-glycosylated threonine derivatives followed by acylation with Fmoc-OSu and deacetylation with methanolic hydrazine yielded Fmoc-Thr(beta-Glc)-OH and Fmoc-Thr(beta-Gal)-OH, respectively. The O-glycosylated threonine derivatives were then reacted with H-Lys(Z)-Pro-Arg(NO2)-OBzl in the presence of DCC and HOBt and the resulting glycosylated tuftsin derivatives were fully deblocked by catalytic hydrogenation, purified by HPLC, and characterized by optical rotation, amino acid analysis, and 1H NMR. The beta-galactosylated tuftsin was also prepared by the continuous flow solid phase procedure.


