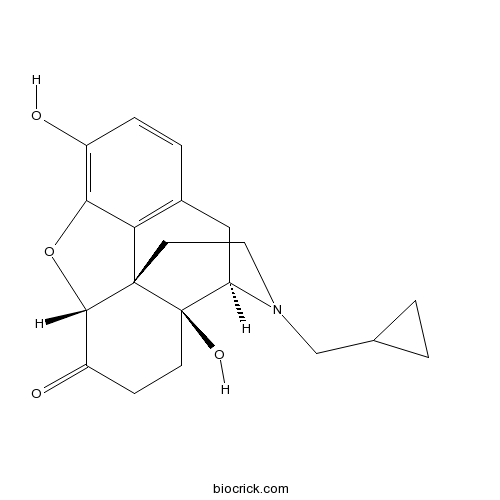
NaltrexoneOpioid receptor antagonist CAS# 16590-41-3 |
- Alvimopan monohydrate
Catalog No.:BCC1349
CAS No.:1383577-62-5
- Alvimopan
Catalog No.:BCC1347
CAS No.:156053-89-3
- Alvimopan dihydrate
Catalog No.:BCC1348
CAS No.:170098-38-1
- ADL5859 HCl
Catalog No.:BCC1265
CAS No.:850173-95-4
- Cebranopadol
Catalog No.:BCC1467
CAS No.:863513-91-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 16590-41-3 | SDF | Download SDF |
| PubChem ID | 5360515 | Appearance | Powder |
| Formula | C20H23NO4 | M.Wt | 341.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO | ||
| Chemical Name | (4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-dihydroxy-2,4,5,6,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-7-one | ||
| SMILES | C1CC1CN2CCC34C5C(=O)CCC3(C2CC6=C4C(=C(C=C6)O)O5)O | ||
| Standard InChIKey | DQCKKXVULJGBQN-XFWGSAIBSA-N | ||
| Standard InChI | InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Naltrexone Dilution Calculator

Naltrexone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9291 mL | 14.6456 mL | 29.2912 mL | 58.5823 mL | 73.2279 mL |
| 5 mM | 0.5858 mL | 2.9291 mL | 5.8582 mL | 11.7165 mL | 14.6456 mL |
| 10 mM | 0.2929 mL | 1.4646 mL | 2.9291 mL | 5.8582 mL | 7.3228 mL |
| 50 mM | 0.0586 mL | 0.2929 mL | 0.5858 mL | 1.1716 mL | 1.4646 mL |
| 100 mM | 0.0293 mL | 0.1465 mL | 0.2929 mL | 0.5858 mL | 0.7323 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Naltrexone is an opioid receptor antagonist approved for the treatment of opioid and alcohol dependence and has been under investigation for tobacco dependence. Naltrexone has been shown to reduce urge to smoke and smoking behavior, and increase quit rates during 4 to 12 weeks of active treatment. [1]
There are three types of opioid receptors classified according to their specific ligands, the μ-opioid receptor (OPRM1), the k-opioid receptor (OPRK1) and the σ-opioid receptor (OPRD1). There is a high concentration of μ-opioid receptors in areas of the brain that have been implicated in the reward pathway associated with alcohol and the μ-opioid receptor is increasingly becoming a main focus in genetic studies of alcohol addiction.[2]
Naltrexone is thought to act as a competitive antagonist at mc, κ, and δ receptors in the CNS, with the highest affintiy for the μ receptor. Naltrexone competitively binds to such receptors and may block the effects of endogenous opioids. This leads to the antagonization of most of the subjective and objective effects of opiates, including respiratory depression, miosis, euphoria, and drug craving. The major metabolite of naltrexone, 6-β-naltrexol, is also an opiate antagonist and may contribute to the antagonistic activity of the drug.
In the mouse, rat and guinea pig, the oral LD50s are 1,100-1,550 mg/kg; 1,450 mg/kg; and 1,490 mg/kg; respectively. High doses of naltrexone (generally ≥1,000 mg/kg) produce salivation, reduced activity, tremors, and convulsions. [1] Pharmacokinetic data also indicates that standard oral naltrexone treatment (generally 50 mg), which has shown moderate to good clinical efficacy in the management of alcohol dependence, is likely to result in peak blood concentrations of 8.5 ng/ml and trough concentrations of less than 0.2 ng/ml within 12 h. Higher doses of oral naltrexone (150 mg/day) have been shown to reduce significantly alcohol consumption (percentage of drinking days, P < 0.0001 and number of drinks per drinking day, P < 0.0001) among those with strong craving, without significant adverse effects.[2]
References:
[1] Daniel J. Fridberg, Dingcai Cao, Jon E. Grant, and Andrea C. King. Naltrexone Improves Quit Rates, Attenuates Smoking Urge, and Reduces Alcohol Use in Heavy Drinking Smokers Attempting to Quit Smoking. CLINICAL AND EXPERIMENTAL RESEARCH Vol. 38, No. 10 October 2014.
[2] Gary K.Hulse. Improving clinical outcomes for naltrexone as a management of problem alcohol use. British Journal of Clinical Pharmacology. DOI:10.1111/j.1365-2125.2012.04452.x
- 1-Hydroxy-N-methylacridone
Catalog No.:BCN7551
CAS No.:16584-54-6
- Eperezolid
Catalog No.:BCC5177
CAS No.:165800-04-4
- Linezolid
Catalog No.:BCC2496
CAS No.:165800-03-3
- Torososide A
Catalog No.:BCN4694
CAS No.:165689-32-7
- Methyl 6-acetoxyangolensate
Catalog No.:BCN1732
CAS No.:16566-88-4
- L-Stepholidine
Catalog No.:BCN2599
CAS No.:16562-13-3
- 12-O-tetradecanoylphorbol-13-acetate
Catalog No.:BCN2511
CAS No.:16561-29-8
- DEPBT
Catalog No.:BCC2811
CAS No.:165534-43-0
- Di-Dnp-L-Lysine
Catalog No.:BCC8939
CAS No.:1655-49-8
- 16-Hydroxy-2-oxocleroda-3,13-dien-15,16-olide
Catalog No.:BCN1536
CAS No.:165459-53-0
- bis[6-(5,6-dihydrochelerythrinyl)]amine
Catalog No.:BCN8232
CAS No.:165393-48-6
- SA 4503 dihydrochloride
Catalog No.:BCC6339
CAS No.:165377-44-6
- Calystegine A5
Catalog No.:BCN1887
CAS No.:165905-26-0
- (-)-Tetramisole
Catalog No.:BCC4670
CAS No.:16595-80-5
- Z-Thr-OBzl
Catalog No.:BCC2737
CAS No.:16597-50-5
- Cyclogrifolin
Catalog No.:BCN7547
CAS No.:1660156-04-6
- 3,4-O-dimethylcedrusin
Catalog No.:BCN8211
CAS No.:166021-14-3
- Ginkgolic acid C15:0
Catalog No.:BCN2483
CAS No.:16611-84-0
- CCT251545
Catalog No.:BCC6487
CAS No.:1661839-45-7
- Bathophenanthroline
Catalog No.:BCC8840
CAS No.:1662-01-7
- Argentinine
Catalog No.:BCN3987
CAS No.:16625-57-3
- Taberdivarine H
Catalog No.:BCN6958
CAS No.:1662688-34-7
- 9-O-Feruloyl-5,5'-dimethoxylariciresinol
Catalog No.:BCN1535
CAS No.:166322-14-1
- m-Nitrohydrocinnamic acid
Catalog No.:BCC9048
CAS No.:1664-57-9
Naltrexone moderates the relationship between cue-induced craving and subjective response to methamphetamine in individuals with methamphetamine use disorder.[Pubmed:28357460]
Psychopharmacology (Berl). 2017 Jul;234(13):1997-2007.
RATIONALE: Reductions in cue-induced craving and subjective response to drugs of abuse are commonly used as initial outcome measures when testing novel medications for the treatment of addiction. Yet neither the relationship between these two measures at the individual level nor the moderating effects of pharmacotherapies on this relationship has been examined. OBJECTIVE: This secondary data analysis sought to examine (1) the predictive relationship between cue-induced craving and subsequent acute subjective response to methamphetamine (MA) and (2) whether the opioid-receptor antagonist Naltrexone moderated this association in a sample of non-treatment-seeking individuals who met DSM-IV criteria for MA use disorder (abuse or dependence). METHODS: Participants (n = 30) completed two 4-day medication regimens (oral Naltrexone 50 mg or placebo, in a randomized, counterbalanced, and double-blind fashion). On day 4 of each medication regimen, participants completed a cue-reactivity paradigm followed by intravenous MA administration. Methamphetamine craving was assessed after the cue-reactivity paradigm, and subjective response to MA was assessed during MA infusion. RESULTS: Cue-induced craving for MA was positively associated with post-infusion subjective MA effects, including positive (i.e., stimulation, good effects, feel drug, high), negative (i.e., anxious and depressed), and craving-related (i.e., want more, would like access to drug, crave) responses. Naltrexone, vs. placebo, significantly reduced the association between cue-induced craving and positive subjective response to MA. CONCLUSIONS: The findings indicate that Naltrexone moderates the predictive relationship between cue-induced craving and positive subjective effects of MA, thereby suggesting a behavioral mechanism by which Naltrexone may be efficacious in treating MA use disorder.
Open-label Study of Injectable Extended-release Naltrexone (XR-NTX) in Healthcare Professionals With Opioid Dependence.[Pubmed:28358754]
J Addict Med. 2017 May/Jun;11(3):224-230.
OBJECTIVES: Healthcare professionals (HCPs) with opioid dependence are at risk for relapse and death, particularly in the first year of recovery; however, maintenance treatment with opioid agonists is controversial in this safety-sensitive group. We evaluated long-term safety, tolerability, and treatment outcomes of injectable, intramuscular, extended-release Naltrexone (XR-NTX) in opioid-dependent HCPs. METHODS: This single-arm, multisite, open-label study was conducted in opioid-dependent HCPs who had been detoxified from opioids for at least 2 weeks. Subjects received monthly XR-NTX injections for up to 24 months, combined with counseling via intensive outpatient substance abuse treatment programs. Assessments included monthly urine opioid drug tests and routine safety assessments, along with a trimonthly short form (36) Health Survey, opioid craving questionnaire, and Treatment Satisfaction Questionnaire for Medication. RESULTS: Of 49 opioid-dependent HCPs screened, 38 enrolled and received at least 1 XR-NTX injection. Most were female (n = 31) and nurses or nursing assistants (n = 30). More than half (n = 21; 55.3%) received at least 12 injections. Seven discontinued due to adverse events (3 anxiety, 2 headache, 1 injection-site mass, 1 derealization). None experienced relapses to opioid dependence necessitating detoxification, overdose, or death during treatment. At 24 months, mean opioid craving fell by 45.2%, and short form (36) mental component scores improved by 31.1% from baseline and approached normal levels. Of 22 unemployed subjects at baseline, 45.5% improved employment status at 24 months. CONCLUSIONS: Long-term (2 years) XR-NTX was associated with no new safety concerns, and, compared with shorter-term studies in the general population, similar or better rates of retention, opioid-negative urines, opioid craving reduction, mental health functional quality of life improvement, and re-employment.
Low-dose naltrexone and opioid consumption: a drug utilization cohort study based on data from the Norwegian prescription database.[Pubmed:28370746]
Pharmacoepidemiol Drug Saf. 2017 Jun;26(6):685-693.
PURPOSE: Low-dose Naltrexone (LDN) is used in a wide range of conditions, including chronic pain and fibromyalgia. Because of the opioid antagonism of Naltrexone, LDN users are probably often warned against concomitant use with opioids. In this study, based on data from the Norwegian prescription database, we examine changes in opioid consumption after starting LDN therapy. METHODS: We included all Norwegian patients (N = 3775) with at least one recorded LDN prescription in 2013 and at least one dispensed opioid prescription during the 365 days preceding the first LDN prescription. We allocated the patients into three subgroups depending on the number of collected LDN prescriptions and recorded the number of defined daily doses (DDDs) on collected prescriptions on opioids, nonsteroidal anti-inflammatory drugs and other analgesics and antipyretics from the same patients. RESULTS: Among the patients collecting >/=4 LDN prescriptions, annual average opioid consumption was reduced by 41 DDDs per person (46%) compared with that of the previous year. The reduction was 12 DDDs per person (15%) among users collecting two to three prescriptions and no change among those collecting only one LDN prescription. We observed no increase in the number of DDDs in nonsteroidal anti-inflammatory drugs or other analgesics and antipyretics corresponding to the decrease in opioid use. CONCLUSIONS: Possibly, LDN users avoided opioids because of warnings on concomitant use or the patients continuing on LDN were less opioid dependent than those terminating LDN. Therapeutic effects of LDN contributing to lower opioid consumption cannot be ruled out. (c) 2017 The Authors. Pharmacoepidemiology & Drug Safety Published by John Wiley & Sons Ltd.


