H-Tyr(Bzl)-OHCAS# 16652-64-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 16652-64-5 | SDF | Download SDF |
| PubChem ID | 86047 | Appearance | Powder |
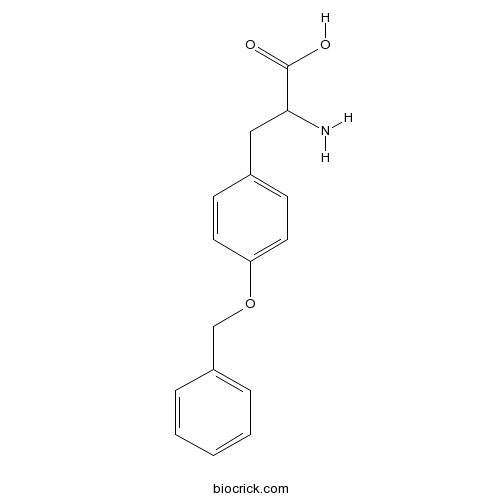
| Formula | C16H17NO3 | M.Wt | 271.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-amino-3-(4-phenylmethoxyphenyl)propanoic acid | ||
| SMILES | C1=CC=C(C=C1)COC2=CC=C(C=C2)CC(C(=O)O)N | ||
| Standard InChIKey | KAFHLONDOVSENM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H17NO3/c17-15(16(18)19)10-12-6-8-14(9-7-12)20-11-13-4-2-1-3-5-13/h1-9,15H,10-11,17H2,(H,18,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

H-Tyr(Bzl)-OH Dilution Calculator

H-Tyr(Bzl)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.686 mL | 18.4298 mL | 36.8596 mL | 73.7191 mL | 92.1489 mL |
| 5 mM | 0.7372 mL | 3.686 mL | 7.3719 mL | 14.7438 mL | 18.4298 mL |
| 10 mM | 0.3686 mL | 1.843 mL | 3.686 mL | 7.3719 mL | 9.2149 mL |
| 50 mM | 0.0737 mL | 0.3686 mL | 0.7372 mL | 1.4744 mL | 1.843 mL |
| 100 mM | 0.0369 mL | 0.1843 mL | 0.3686 mL | 0.7372 mL | 0.9215 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
H-Tyr(Bzl)-OH
- Avasimibe
Catalog No.:BCC2274
CAS No.:166518-60-1
- Metaxalone
Catalog No.:BCC5223
CAS No.:1665-48-1
- trans-4-(Trifluoromethyl)cinnamic acid
Catalog No.:BCN1534
CAS No.:16642-92-5
- p-Nitrohydrocinnamic acid
Catalog No.:BCC9124
CAS No.:16642-79-8
- 1H-Pyrido[3,4-b]indole-1,3 4(2H,9H)-trione
Catalog No.:BCN7913
CAS No.:16641-79-5
- m-Nitrohydrocinnamic acid
Catalog No.:BCC9048
CAS No.:1664-57-9
- 9-O-Feruloyl-5,5'-dimethoxylariciresinol
Catalog No.:BCN1535
CAS No.:166322-14-1
- Taberdivarine H
Catalog No.:BCN6958
CAS No.:1662688-34-7
- Argentinine
Catalog No.:BCN3987
CAS No.:16625-57-3
- Bathophenanthroline
Catalog No.:BCC8840
CAS No.:1662-01-7
- CCT251545
Catalog No.:BCC6487
CAS No.:1661839-45-7
- Ginkgolic acid C15:0
Catalog No.:BCN2483
CAS No.:16611-84-0
- H-Pro-OBzl.HCl
Catalog No.:BCC3021
CAS No.:16652-71-4
- H-Val-OBzl.TosOH
Catalog No.:BCC3140
CAS No.:16652-76-9
- Eurycarpin A
Catalog No.:BCN4693
CAS No.:166547-20-2
- Ramelteon
Catalog No.:BCN2183
CAS No.:196597-26-9
- Anidulafungin
Catalog No.:BCC4233
CAS No.:166663-25-8
- 4,4'-Bis(chloromethyl)biphenyl
Catalog No.:BCC8658
CAS No.:1667-10-3
- cis-Mulberroside A
Catalog No.:BCN3911
CAS No.:166734-06-1
- H-Cys(Trt)-NH2
Catalog No.:BCC2912
CAS No.:166737-85-5
- Naltrexone HCl
Catalog No.:BCC4613
CAS No.:16676-29-2
- Z-Tyr(Bzl)-OH
Catalog No.:BCC2735
CAS No.:16677-29-5
- Prerubialatin
Catalog No.:BCN6895
CAS No.:1667718-89-9
- Desmopressin
Catalog No.:BCC1525
CAS No.:16679-58-6
A structure-activity relationship study and combinatorial synthetic approach of C-terminal modified bifunctional peptides that are delta/mu opioid receptor agonists and neurokinin 1 receptor antagonists.[Pubmed:18266313]
J Med Chem. 2008 Mar 13;51(5):1369-76.
A series of bifunctional peptides with opioid agonist and substance P antagonist bioactivities were designed with the concept of overlapping pharmacophores. In this concept, the bifunctional peptides were expected to interact with each receptor separately in the spinal dorsal horn where both the opioid receptors and the NK1 receptors were found to be expressed, to show an enhanced analgesic effect, no opioid-induced tolerance, and to provide better compliance than coadministration of two drugs. Compounds were synthesized using a two-step combinatorial method for C-terminal modification. In the method, the protected C-terminal-free carboxyl peptide, Boc-Tyr( tBu)- d-Ala-Gly Phe-Pro-Leu-Trp(Boc)-OH, was synthesized as a shared intermediate using Fmoc solid phase chemistry on a 2-chlorotrityl resin. This intermediate was esterified or amidated in solution phase. The structure-activity relationships (SAR) showed that the C-terminus acted as not only a critical pharmacophore for the substance P antagonist activities, but as an address region for the opioid agonist pharmacophore that is structurally distant from the C-terminal. Among the peptides, H-Tyr- d -Ala-Gly-Phe-Pro-Leu-Trp-NH-Bzl ( 3) demonstrated high binding affinities at both delta and mu receptors ( K i = 10 and 0.65 nM, respectively) with efficient agonist functional activity in the mouse isolated vas deferens (MVD) and guinea pig isolated ileum (GPI) assays (IC 50 = 50 and 13 nM, respectively). Compound 3 also showed a good antagonist activity in the GPI assay with substance P stimulation ( K e = 26 nM) and good affinity for the hNK1 receptor ( K i = 14 nM). Consequently, compound 3 is expected to be a promising and novel type of analgesic with bifunctional activities.
Effect of aromatic amino acid substitutions in the 3-position of cyclic beta-casomorphin analogues on mu-opioid agonist/delta-opioid antagonist properties.[Pubmed:8956074]
Int J Pept Protein Res. 1996 Nov;48(5):411-9.
The beta-casomorphin-5 analog H-Tyr-c[-D-Orn-2-Nal-D-Pro-Gly-] (2-Nal = 2-naphthylalanine) was the first reported cyclic opioid peptide with mixed mu agonist/delta antagonist properties [R. Schmidt et al. (1994) J. Med. Chem. 37, 1136-1144]. The 2-Nal3 residue in this peptide was replaced with benzothienylalanine (Bta) (3), His(Bzl) (4), Tyr(Bzl) (5), 4'-benzoylphenylalanine (Bpa) (6), 4'-benzylphenylalanine (Bzp) (7), thyronine (Thy) (8), thyroxine (Thx) (9), 4'-biphenylalanine (Bip) (10), 4'-biphenylglycine (Bpg) (12) and 3,3-diphenylalanine (Dip) (14), and the in vitro opioid activity profiles of the resulting compounds were determined in mu and delta receptor-representative binding assays and bioassays. Analogues 3, 12 and 14 were full agonists in the mu receptor-representative guinea-pig ileum (GPI) assay and also were agonists in the delta receptor-representative mouse vas deferens (MVD) assay. The agonist effects of the latter compounds in the MVD assay were antagonized by the highly selective delta antagonist H-Tyr-Tic-Phe-Phe-OH (TIPP), indicating that they were triggered by delta receptor activation. The Bzp3- and Bip3- containing peptides 7 and 10 turned out to be mu antagonists against the mu selective agonist H-Tyr-D-Ala-Phe-Phe-NH2 in the GPI assay. The other analogues were weak partial mu agonists which displayed remarkably decreased mu receptor affinity as compared to parent peptide 1. Compounds 4-10 were found to be delta antagonists in the MVD assay. Analogues 4 and 9 exhibited delta antagonist potency similar to that of parent peptide 1, while compounds 5-8 and 10 showed 3-12-fold higher delta antagonist potency against DPDPE and deltorphin I and, in most cases, increased delta receptor affinity. These results indicate that the delta receptor tolerates bulky aromatic side chains in the 3-position of cyclic beta-casomorphin analogs with either delta agonist or delta antagonist properties. However, these compounds displayed drastically reduced mu receptor affinity in nearly all cases.


