Avenanthramide DCAS# 115610-36-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 115610-36-1 | SDF | Download SDF |
| PubChem ID | 6443019 | Appearance | White-pale yellow powder |
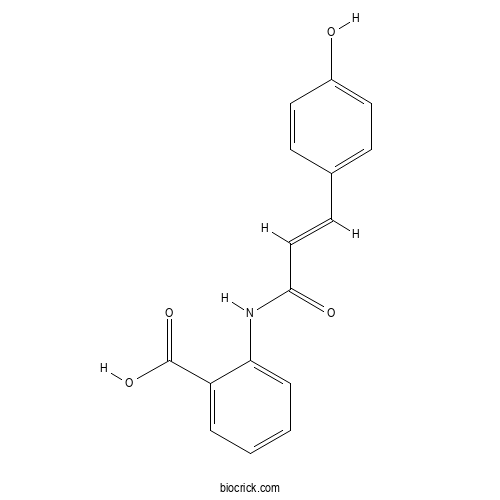
| Formula | C16H13NO4 | M.Wt | 283.28 |
| Type of Compound | Nitrogen-containing Compounds | Storage | Desiccate at -20°C |
| Synonyms | Avenanthramide 1p; N-p-Coumaroylanthranilic acid; Dianthramide P | ||
| Solubility | Soluble in methanol; Slightly soluble in acetone and acetonitrile; insoluble in water | ||
| Chemical Name | 2-[[(E)-3-(4-hydroxyphenyl)prop-2-enoyl]amino]benzoic acid | ||
| SMILES | C1=CC=C(C(=C1)C(=O)O)NC(=O)C=CC2=CC=C(C=C2)O | ||
| Standard InChIKey | INBHLTYBRKASIZ-JXMROGBWSA-N | ||
| Standard InChI | InChI=1S/C16H13NO4/c18-12-8-5-11(6-9-12)7-10-15(19)17-14-4-2-1-3-13(14)16(20)21/h1-10,18H,(H,17,19)(H,20,21)/b10-7+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Avenanthramide D Dilution Calculator

Avenanthramide D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5301 mL | 17.6504 mL | 35.3008 mL | 70.6015 mL | 88.2519 mL |
| 5 mM | 0.706 mL | 3.5301 mL | 7.0602 mL | 14.1203 mL | 17.6504 mL |
| 10 mM | 0.353 mL | 1.765 mL | 3.5301 mL | 7.0602 mL | 8.8252 mL |
| 50 mM | 0.0706 mL | 0.353 mL | 0.706 mL | 1.412 mL | 1.765 mL |
| 100 mM | 0.0353 mL | 0.1765 mL | 0.353 mL | 0.706 mL | 0.8825 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Atropine N-oxide hydrochloride
Catalog No.:BCN0300
CAS No.:4574-60-1
- Apoatropine hydrochloride
Catalog No.:BCN0299
CAS No.:5978-81-4
- (-)-Antofine
Catalog No.:BCN0298
CAS No.:32671-82-2
- Anisodine hydrobromide
Catalog No.:BCN0297
CAS No.:76822-34-9
- Anemonin
Catalog No.:BCN0296
CAS No.:508-44-1
- Andrograpanin
Catalog No.:BCN0295
CAS No.:82209-74-3
- L-(±)-Alliin
Catalog No.:BCN0294
CAS No.:17795-26-5
- Procyanidin A4
Catalog No.:BCN0293
CAS No.:111466-29-6
- Ergocornine
Catalog No.:BCN0292
CAS No.:564-36-3
- Agroclavine
Catalog No.:BCN0291
CAS No.:548-42-5
- Roridin A
Catalog No.:BCN0290
CAS No.:14729-29-4
- Incarvilone A
Catalog No.:BCN0286
CAS No.:
- Bakuchicin
Catalog No.:BCN0302
CAS No.:4412-93-5
- Ballonigrine
Catalog No.:BCN0303
CAS No.:62340-62-9
- Berberine chloride dihydrate
Catalog No.:BCN0304
CAS No.:5956-60-5
- Californidine perchlorate
Catalog No.:BCN0305
CAS No.:17939-31-0
- Cannflavin A
Catalog No.:BCN0306
CAS No.:76735-57-4
- Castalagin
Catalog No.:BCN0307
CAS No.:24312-00-3
- (-)-Catechin
Catalog No.:BCN0308
CAS No.:18829-70-4
- Convolidine
Catalog No.:BCN0309
CAS No.:63911-32-0
- trans-p-Coumaric acid
Catalog No.:BCN0310
CAS No.:501-98-4
- trans-Coutaric acid
Catalog No.:BCN0311
CAS No.:27174-07-8
- Cycloartenol
Catalog No.:BCN0312
CAS No.:469-38-5
- 5,6-Dehydro 7,8-dihydrokavain
Catalog No.:BCN0313
CAS No.:3155-51-9
Dihydroavenanthramide D prevents UV-irradiated generation of reactive oxygen species and expression of matrix metalloproteinase-1 and -3 in human dermal fibroblasts.[Pubmed:24103002]
Exp Dermatol. 2013 Nov;22(11):759-61.
Ultraviolet B (UVB) radiation induces photoageing by upregulating the expression of matrix metalloproteinases (MMPs) in human skin cells. DihydroAvenanthramide D (DHAvD) is a synthetic analog to naturally occurring avenanthramide, which is the active component in oats. Although anti-inflammatory, anti-atherosclerotic and antioxidant effects have been reported, the antiphotoageing effects of DHAvD are yet to be understood. In this study, we investigated the inhibitory effects of DHAvD on UVB-induced production of reactive oxygen species (ROS) and expression of MMPs, and its molecular mechanism in UVB-irradiated human dermal fibroblasts. Western blot and real-time PCR analyses revealed that DHAvD inhibited UVB-induced MMP-1 and MMP-3 expression. It also significantly blocked UVB-induced ROS generation in fibroblasts. Additionally, DHAvD attenuated UVB-induced phosphorylation of MAPKs, activation of NF-kappaB and AP-1. DHAvD regulates UVB-irradiated MMP expression by inhibiting ROS-mediated MAPK/NF-kappaB and AP-1 activation. DHAvD may be a useful candidate for preventing UV light-induced skin photoageing.
Dihydroavenanthramide D inhibits human breast cancer cell invasion through suppression of MMP-9 expression.[Pubmed:21262201]
Biochem Biophys Res Commun. 2011 Feb 25;405(4):552-7.
DihydroAvenanthramide D (DHAvD) is a synthetic analog to naturally occurring avenanthramide, which is the active component of oat. Previous study demonstrates that DHAvD strongly inhibits activation of nuclear factor-kappa B (NF-kappaB), which is a major component in cancer cell invasion. The present study investigated whether DHAvD can modulate MMP-9 expression and cell invasion in MCF-7 human breast cancer cells. MMP-9 expression and cell invasion in response to 12-O-tetradecanoylphorbol-13-acetate (TPA) was increased, whereas these inductions were muted by DHAvD. DHAvD also suppressed activation of mitogen-activated protein kinase (MAPK), and MAPK-mediated nuclear factor-kappa B (NF-kappaB) and activator protein-1 (AP-1) activations in TPA-treated MCF-7 cells. The results indicate that DHAvD-mediated inhibition of TPA-induced MMP-9 expression and cell invasion involves the suppression of the MAPK/NF-kappaB and MAPK/AP-1 pathways in MCF-7 cells. DHAvD may have potential value in breast cancer metastasis.


