AndrograpaninCAS# 82209-74-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

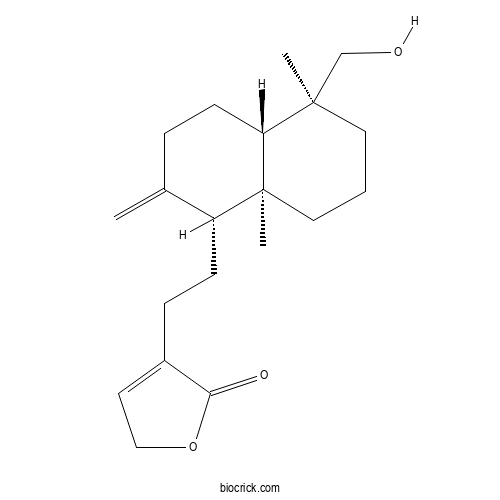
| Cas No. | 82209-74-3 | SDF | Download SDF |
| PubChem ID | 134688657 | Appearance | White powder |
| Formula | C20H30O3 | M.Wt | 318.4 |
| Type of Compound | Isoprenoids | Storage | Desiccate at -20°C |
| Synonyms | 3,14-Dideoxyandrographolide | ||
| Solubility | Soluble in chloroform and methanol; insoluble in water | ||
| Chemical Name | 4-[2-[(1R,4aS,5S,8aS)-5-(hydroxymethyl)-5,8a-dimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1H-naphthalen-1-yl]ethyl]-2H-furan-5-one | ||
| SMILES | CC1(CCCC2(C1CCC(=C)C2CCC3=CCOC3=O)C)CO | ||
| Standard InChIKey | WKKBRRFSRMDTJB-LFGUQSLTSA-N | ||
| Standard InChI | InChI=1S/C20H30O3/c1-14-5-8-17-19(2,13-21)10-4-11-20(17,3)16(14)7-6-15-9-12-23-18(15)22/h9,16-17,21H,1,4-8,10-13H2,2-3H3/t16-,17-,19-,20+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Andrograpanin Dilution Calculator

Andrograpanin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1407 mL | 15.7035 mL | 31.407 mL | 62.8141 mL | 78.5176 mL |
| 5 mM | 0.6281 mL | 3.1407 mL | 6.2814 mL | 12.5628 mL | 15.7035 mL |
| 10 mM | 0.3141 mL | 1.5704 mL | 3.1407 mL | 6.2814 mL | 7.8518 mL |
| 50 mM | 0.0628 mL | 0.3141 mL | 0.6281 mL | 1.2563 mL | 1.5704 mL |
| 100 mM | 0.0314 mL | 0.157 mL | 0.3141 mL | 0.6281 mL | 0.7852 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- L-(±)-Alliin
Catalog No.:BCN0294
CAS No.:17795-26-5
- Procyanidin A4
Catalog No.:BCN0293
CAS No.:111466-29-6
- Ergocornine
Catalog No.:BCN0292
CAS No.:564-36-3
- Agroclavine
Catalog No.:BCN0291
CAS No.:548-42-5
- Roridin A
Catalog No.:BCN0290
CAS No.:14729-29-4
- Incarvilone A
Catalog No.:BCN0286
CAS No.:
- Incarvine F
Catalog No.:BCN0285
CAS No.:
- Incarvine E
Catalog No.:BCN0284
CAS No.:
- Incarvine D
Catalog No.:BCN0283
CAS No.:
- Incarvine C
Catalog No.:BCN0282
CAS No.:
- Incarvine A
Catalog No.:BCN0281
CAS No.:
- Vatalbinoside F
Catalog No.:BCN0280
CAS No.:
- Anemonin
Catalog No.:BCN0296
CAS No.:508-44-1
- Anisodine hydrobromide
Catalog No.:BCN0297
CAS No.:76822-34-9
- (-)-Antofine
Catalog No.:BCN0298
CAS No.:32671-82-2
- Apoatropine hydrochloride
Catalog No.:BCN0299
CAS No.:5978-81-4
- Atropine N-oxide hydrochloride
Catalog No.:BCN0300
CAS No.:4574-60-1
- Avenanthramide D
Catalog No.:BCN0301
CAS No.:115610-36-1
- Bakuchicin
Catalog No.:BCN0302
CAS No.:4412-93-5
- Ballonigrine
Catalog No.:BCN0303
CAS No.:62340-62-9
- Berberine chloride dihydrate
Catalog No.:BCN0304
CAS No.:5956-60-5
- Californidine perchlorate
Catalog No.:BCN0305
CAS No.:17939-31-0
- Cannflavin A
Catalog No.:BCN0306
CAS No.:76735-57-4
- Castalagin
Catalog No.:BCN0307
CAS No.:24312-00-3
Molecular docking analysis of compounds from Andrographis paniculata with EGFR.[Pubmed:34393414]
Bioinformation. 2021 Jan 31;17(1):23-28.
EGFR is linked with oral cancer. Therefore, it is of interest document the molecular docking analysis of compounds from Andrographis paniculata with EGFR. Data shows the binding features of five compounds 14- acetylandrographolide, Andrograpanin, andrographolide, isoandrographolide and neoandrographolide from Andrographis paniculata with EGFR for further consideration.
UGT86C11 is a novel plant UDP-glycosyltransferase involved in labdane diterpene biosynthesis.[Pubmed:34363833]
J Biol Chem. 2021 Aug 4;297(3):101045.
Glycosyltransferases constitute a large family of enzymes across all domains of life, but knowledge of their biochemical function remains largely incomplete, particularly in the context of plant specialized metabolism. The labdane diterpenes represent a large class of phytochemicals with many pharmacological benefits, such as anti-inflammatory, hepatoprotective, and anticarcinogenic. The medicinal plant kalmegh (Andrographis paniculata) produces bioactive labdane diterpenes; notably, the C19-hydroxyl diterpene (Andrograpanin) is predominantly found as C19-O-glucoside (neoandrographolide), whereas diterpenes having additional hydroxylation(s) at C3 (14-deoxy-11,12-didehydroandrographolide) or C3 and C14 (andrographolide) are primarily detected as aglycones, signifying scaffold-selective C19-O-glucosylation of diterpenes in planta. Here, we analyzed UDP-glycosyltransferase (UGT) activity and diterpene levels across various developmental stages and tissues and found an apparent correlation of UGT activity with the spatiotemporal accumulation of neoandrographolide, the major diterpene C19-O-glucoside. The biochemical analysis of recombinant UGTs preferentially expressed in neoandrographolide-accumulating tissues identified a previously uncharacterized UGT86 member (ApUGT12/UGT86C11) that catalyzes C19-O-glucosylation of diterpenes with strict scaffold selectivity. ApUGT12 localized to the cytoplasm and catalyzed diterpene C19-O-glucosylation in planta. The substrate selectivity demonstrated by the recombinant ApUGT12 expressed in plant and bacterium hosts was comparable to native UGT activity. Recombinant ApUGT12 showed significantly higher catalytic efficiency using Andrograpanin compared with 14-deoxy-11,12-didehydroandrographolide and trivial activity using andrographolide. Moreover, ApUGT12 silencing in plants led to a drastic reduction in neoandrographolide content and increased levels of Andrograpanin. These data suggest the involvement of ApUGT12 in scaffold-selective C19-O-glucosylation of labdane diterpenes in plants. This knowledge of UGT86 function might help in developing plant chemotypes and synthesis of pharmacologically relevant diterpenes.
In vitro and in silico studies on the structural and biochemical insight of anti-biofilm activity of andrograpanin from Andrographis paniculata against Pseudomonas aeruginosa.[Pubmed:32851551]
World J Microbiol Biotechnol. 2020 Aug 27;36(10):143.
Microbial infections have become a global threat to drug-tolerant phenomena due to their biofilm formatting capacity. In many cases, conventional antimicrobial drugs fail to combat the infection, thus necessitating the discovery of some alternative medicine. Over several decades, plant metabolites have played a critical role in treating a broad spectrum of microbial infections due to its low cytotoxicity. Andrograpanin, a secondary metabolite, is a diterpenoid present in the leaf of Andrographis paniculata. In this study, Andrograpanin (0.15 mM) exhibited significant inhibition on biofilm production by Pseudomonas aeruginosa in the presence of gentamicin (0.0084 mM). The impaired production of extracellular polymeric substances and several virulence factors of Pseudomonas aeruginosa were investigated to understand the mechanism of action mediated by Andrograpanin. The structural alteration of biofilm was evaluated by using fluorescence microscopy, atomic force microscopy and field emission scanning electron microscopy. The in silico molecular simulation studies predicted interaction of Andrograpanin with quorum sensing proteins such as RhlI, LasI, LasR, and swarming motility protein BswR of Pseudomonas aeruginosa. Overall the studies indicate that Andrograpanin could be used as a therapeutic molecule against biofilm development by Pseudomonas aeruginosa.
Diterpenoids and Flavonoids from Andrographis paniculata.[Pubmed:31902905]
Chem Pharm Bull (Tokyo). 2020;68(1):96-99.
Chemical investigation of the aerial parts of Andrographis paniculata resulted in isolation of nine compounds, including a new ent-labdane diterpenoid, andrographic acid methyl ester (1), a new chalcone glucoside, pashanone glucoside (5), and seven known metabolites, Andrograpanin (2), andrographolide (3), andropanolide (4), andrographidine A (6), andrographidine F (7), 6-epi-8-O-acetyl-harpagide (8), and curvifloruside F (9). Their chemical structures were elucidated based on comprehensive analyses of the spectroscopic data, including NMR and MS. Among the isolated compounds, andropanolide exerted cytotoxicity toward LNCaP, HepG2, KB, MCF7, and SK-Mel2 carcinoma cells, with IC50 values ranging from 31.8 to 45.9 microM. In addition, andropanolide significantly inhibited the overproduction of nitric oxide (NO) in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages, with an IC50 value of 13.4 microM.
Ovicidal and larvicidal effects of extracts from leaves of Andrographis paniculata (Burm. f.) Wall.ex Nees against field isolates of human hookworm (Ancylostoma duodenale).[Pubmed:30763693]
J Ethnopharmacol. 2019 May 10;235:489-500.
ETHNOPHARMACOLOGICAL RELEVANCE: The whole plant of Andrographis paniculata (Burm. f.) Wall.ex Nees is used traditionally in different forms by the local people of Asian countries owing to its myriad medicinal properties. Its use as an anthelmintic has been mentioned in literature but has not been well elucidated. AIM OF THE STUDY: To determine anthelmintic effects of extracts from leaves of A.paniculata against human hookworm species based on a standard assay system and to establish the effects of major active compounds responsible for the effects. MATERIALS AND METHODS: Ovicidal and larvicidal activities of extracts of leaves of A.paniculata in different solvents ethanol (Et), methanol (Met), ethyl acetate (EA) and petroleum ether (PE) was studied against field isolates of Ancylostoma duodenale collected and cultivated from hookworm infected human stool samples by egg hatch and larval motility assays. Major active compounds namely andrographolide (AP1), neoandrographolide (AP2) and Andrograpanin (AP3) were estimated quantitatively in all the extracts by high-performance liquid chromatography (HPLC) and mass spectrometry (MS) analysis. Anthelmintic effects (ED50, LC50) and presence of the marker compounds in each extract was statistically analyzed by principal component analysis (PCA). Further, biological activities of pure compounds of AP1, AP2, AP3 were assessed to validate the results of the study. RESULTS: Extracts in ethanol and methanol showed highest activity in inhibition of egg hatching with lowest ED50 values (0.017 and 0.02mg/mL respectively) while ethyl acetate extract had the highest activity against larval motility (0.001mg/mL) followed by ethanol (0.019mg/mL). On HPLC analysis, andrographolide content (%), the major diterpene compound, in Met and Et was 0.85 and 1.43 respectively. On PCA, andrographolide component in the extracts was associated with significant inhibitory effects both on egg hatching and larval motility. Pure compound AP1 also showed significant ovicidal and larvicidal activities at concentrations 0.125microg/mL and 0.019mg/mL respectively. CONCLUSION: Andrographolide is one of the main phytochemical responsible for significant ovicidal and larvicidal activity against field isolates of A.duodenale from human infections and can be developed as a potential therapeutic choice.
The genome of the medicinal plant Andrographis paniculata provides insight into the biosynthesis of the bioactive diterpenoid neoandrographolide.[Pubmed:30444296]
Plant J. 2019 Mar;97(5):841-857.
Andrographis paniculata is a herbaceous dicot plant widely used for its anti-inflammatory and anti-viral properties across its distribution in China, India and other Southeast Asian countries. A. paniculata was used as a crucial therapeutic treatment during the influenza epidemic of 1919 in India, and is still used for the treatment of infectious disease in China. A. paniculata produces large quantities of the anti-inflammatory diterpenoid lactones andrographolide and neoandrographolide, and their analogs, which are touted to be the next generation of natural anti-inflammatory medicines for lung diseases, hepatitis, neurodegenerative disorders, autoimmune disorders and inflammatory skin diseases. Here, we report a chromosome-scale A. paniculata genome sequence of 269 Mb that was assembled by Illumina short reads, PacBio long reads and high-confidence (Hi-C) data. Gene annotation predicted 25 428 protein-coding genes. In order to decipher the genetic underpinning of diterpenoid biosynthesis, transcriptome data from seedlings elicited with methyl jasmonate were also obtained, which enabled the identification of genes encoding diterpenoid synthases, cytochrome P450 monooxygenases, 2-oxoglutarate-dependent dioxygenases and UDP-dependent glycosyltransferases potentially involved in diterpenoid lactone biosynthesis. We further carried out functional characterization of pairs of class-I and -II diterpene synthases, revealing the ability to produce diversified labdane-related diterpene scaffolds. In addition, a glycosyltransferase able to catalyze O-linked glucosylation of Andrograpanin, yielding the major active product neoandrographolide, was also identified. Thus, our results demonstrate the utility of the combined genomic and transcriptomic data set generated here for the investigation of the production of the bioactive diterpenoid lactone constituents of the important medicinal herb A. paniculata.
Glucosyltransferase Capable of Catalyzing the Last Step in Neoandrographolide Biosynthesis.[Pubmed:30234309]
Org Lett. 2018 Oct 5;20(19):5999-6002.
ApUGT, a diterpene glycosyltransferase from Andrographis paniculata, could transfer a glucose to the C-19 hydroxyl moiety of Andrograpanin to form neoandrographolide. This glycosyltransferase has a broad substrate scope, and it can glycosylate 26 natural and unnatural compounds of different structural types. This study provides a basis for exploring the glycosylation mechanism of ent-labdane-type diterpenes and plays an important role in diversifying the structures used in drug discovery.
[Chemical constituents from Fukeqianjin formula].[Pubmed:29945383]
Zhongguo Zhong Yao Za Zhi. 2018 Jun;43(11):2300-2312.
Fukeqianjin formula, a traditional Chinese medicine compound, consists of eight Chinese medicinal materials including roots of Moghania macrophylla, roots of Rosa laevigata, aerial parts of Andrographis paniculata, caulis of Mahonia fortunei, roots of Zanthoxylum dissitum, roots of Angelica sinensis, caulis of Spatholobus suberectus, and roots of Codonopsis pilosula. The chemical constituents from Fukeqianjin formula were studied in this paper. The compounds were separated and purified by repeated column chromatographic methods including silica gel, Sephadex LH-20, macroporous adsorptive resin, and reverse phase high performance liquid chromatography. And their chemical structures were determined by spectral data analyses. Thirty-eight compounds were obtained and identified as Z-3-butylidenephthalide (1), Z-ligustilide (2), senkyunolide I (3), senkyunolide H (4), vanillin (5), 7-O-methylwogonin (6), wogonin (7), panicolin (8), 19-hydroxy-8(17),13-labdadien-15,16-olide (9), Andrograpanin (3,14-dideoxyandrographolide; 10), andrographolide (11), 14-deoxy-11,12-didehydroandrographolide (12), isoandrographolide (13), andrographin (2'-O-methylskullcapflavone, 14), biochanin A (15), 5-hydroxy-7,8,2',5'-tetramethoxyflavone (16), formononetin (17), daidzein (18), genistein (19), benzoic acid (20), vanillic acid (21), trans-ferulic acid (22), salicylic acid (23), daidzin (24), genistein-7-O-beta-D-apiofuranosyl-(1-->6)-O-beta-D-glucopyranoside (25), apigenin-7-O-beta-D-glucuronide (26), andrographidin C (27), apigenin-7-O-beta-D-(6"-methyl)glucuronide (28), neoandrographolide (29), genistin (30), andrographiside (31), 14-deoxy-11,12-didehydroandrographiside (32), lobetyolin (33), epicatechin (34), catechin (35), palmatine (36), berberine (37), and jatrorrhizine (38), respectively. From the results of an individual medicinal material studies, it can be judged that compounds 17, 19, 24 and 30 as flavonoids came from the roots of M. macrophylla, compounds 36-38 as alkaloids came from the caulis of M. fortunei, compounds 6-8, 14, 16, and 27 as flavonoids as well as 9-13, 29, 31, and 32 as diterpenes came from the aerial parts of A. paniculata, compound 5 as phenols came from the roots of Z. dissitum, compounds 1-4 as phthalides as well as compound 22 as phenylpropanoids came from the roots of A. sinensis, compound 33 as alkynes came from the roots of C. pilosula, compounds 15, 17-19 as flavonoids as well as compound 21 as phenolic acids came from the caulis of S. suberectus. While compounds 34 and 35 as flavanoids could come from both the caulis of S. suberectus and roots of R. laevigata. The chemical composition of traditional Chinese medicine compound can be tracked from the original sources. This work provides a demonstration for the material basis study of traditional Chinese medicine compound. Compounds 25, 26 and 28 have not so far been isolated and identified from the above-mentioned single herb.
Extraction of three bioactive diterpenoids from Andrographis paniculata: effect of the extraction techniques on extract composition and quantification of three andrographolides using high-performance liquid chromatography.[Pubmed:24170124]
J Chromatogr Sci. 2014 Oct;52(9):1043-50.
Andrographis paniculata (Burm.f.) wall.ex Nees (Acanthaceae) or Kalmegh is an important medicinal plant finding uses in many Ayurvedic formulations. Diterpenoid compounds andrographolides (APs) are the main bioactive phytochemicals present in leaves and herbage of A. paniculata. The efficiency of supercritical fluid extraction (SFE) using carbon dioxide was compared with the solid-liquid extraction techniques such as solvent extraction, ultrasound-assisted solvent extraction and microwave-assisted solvent extraction with methanol, water and methanol-water as solvents. Also a rapid and validated reverse-phase high-performance liquid chromatography-diode array detection method was developed for the simultaneous determination of the three biologically active compounds, AP, neoandrographolide and Andrograpanin, in the extracts of A. paniculata. Under the best SFE conditions tested for diterpenoids, which involved extraction at 60 degrees C and 100 bar, the extractive efficiencies were 132 and 22 microg/g for AP and neoandrographolide, respectively. The modifier percentage significantly affected the extraction efficiency.
[Chemical constituents from roots of Andrographis paniculata].[Pubmed:21626787]
Yao Xue Xue Bao. 2011 Mar;46(3):317-21.
To investigate the chemical constituents of the roots of Andrographis paniculata, 28 compounds were isolated and identified from the 80% ethanol extract. There are 20 flavonoids: 5, 5'-dihydroxy-7, 8, 2'-trimetroxyflavone (1), 5-hydroxy-7, 8, 2', 6'-tetramethoxyflavone (2), 5, 3'-dihydroxy-7, 8, 4'-trimethoxyflavone (3), 2'-hydroxy-5, 7, 8-trimethoxyflavone (4), 5-hydroxy-7, 8, 2', 3', 4'-pentamethoxyflavone (6), wightin (7), 5, 2', 6'-trihydroxy-7-methoxyflavone 2'-O-beta-D-glucopyranoside (8), 5, 7, 8, 2'-tetramethoxyflavone (10), 5-hydroxy-7, 8-dimethoxyflavanone (11), 5-hydroxy-7, 8-dimethoxyflavone (12), 5, 2'-dihydroxy-7, 8-dimethoxyflavone (13), 5-hydroxy-7, 8, 2', 5'-tetramethoxyflavone (14), 5-hydroxy-7, 8, 2', 3'-tetramethoxyflavone (15), 5-hydroxy-7, 8, 2'-trimethoxyflavone (16), 5, 4'-dihydroxy-7, 8, 2', 3'-tetramethoxyflavone (17), dihydroneobaicalein (18), andrographidine A (19), andrographidine B (20), andrographidine C (21) and 5, 2'-dihydroxy-7, 8-dimethoxyflavone 2'-O-beta-D-glucopyranoside (22); three diterpenoids: Andrograpanin (23), neoandrographolide (24) and andrographolide (25); two phenylpropanoids: trans-cinnamic acid (26) and 4-hydroxy-2-methoxycinnamaldehyde (5); and oleanolic acid (9), beta-sitosterol (27) and beta-daucosterol (28). Compound 1 is a new flavone, compound 4 is a new natural product, compounds 2, 3 and 5 were isolated from the Androggraphis genus for the first time and compounds 6-9 were isolated from this plant for the first time.
In vitro modulation of LPS/calcimycin induced inflammatory and allergic mediators by pure compounds of Andrographis paniculata (King of bitters) extract.[Pubmed:21034865]
Int Immunopharmacol. 2011 Jan;11(1):79-84.
The aim of the current study is to probe the anti-inflammatory/anti-allergic potential of seven phytoconstituents (andrographolide, neoandrographolide, isoandrographolide, Andrograpanin, 14-deoxy-11,12-didehydroandrographolide, 7-O-methylwogonin and skullcapflavone-I) isolated from Andrographis paniculata (King of bitters) on the production of key inflammatory/allergic mediators (NO, PGE(2), IL-1 beta, IL-6, LTB(4), TXB(2) and histamine). The results demonstrated that andrographolide, isoandrographolide, 7-O-methylwogonin and skullcapflavone-I significantly inhibited LPS stimulated NO and PGE(2) release in J774A.1 macrophages. Andrographolide, isoandrographolide and 7-O-methylwogonin showed considerable inhibition of IL-1 beta production in LPS elicited macrophages. LPS induced IL-6 production was significantly inhibited by andrographolide, isoandrographolide and skullcapflavone-I in a concentration dependent manner. The results revealed that andrographolide, isoandrographolide and skullcapflavone-I significantly decreased TXB(2) release in A23187 activated HL-60 promyelocytic cells. Furthermore, the anti-allergic properties of the phytoconstituents was investigated on A23187 induced LTB(4) production (HL-60 cells) and histamine release (RBL-2H3 basophilic cells). The results showed that only skullcapflavone-I and 7-O-methylwogonin showed marked inhibitory effect on LTB(4) production, however, only 7-O-methylwogonin exerted dose-dependent inhibition towards histamine release. Therefore, this study indicates that some of these phytoconstituents exhibit potent anti-inflammatory/anti-allergic effects by modulating different inflammatory/allergic mediators. Hence, these phytoconstituents might provide useful phytomedical treatment against variety of inflammatory and allergic disorders.
Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian).[Pubmed:20465823]
Chin Med. 2010 May 13;5:17.
Andrographis paniculata (Burm. f.) Nees (Acanthaceae) is a medicinal plant used in many countries. Its major constituents are diterpenoids, flavonoids and polyphenols. Among the single compounds extracted from A. paniculata, andrographolide is the major one in terms of bioactive properties and abundance. Among the andrographolide analogues, 14-deoxy-11,12-didehydroandrographolide is immunostimulatory, anti-infective and anti-atherosclerotic; neoandrographolide is anti-inflammatory, anti-infective and anti-hepatotoxic; 14-deoxyandrographolide is immunomodulatory and anti-atherosclerotic. Among the less abundant compounds from A. paniculata, Andrograpanin is both anti-inflammatory and anti-infective; 14-deoxy-14,15-dehydroandrographolide is anti-inflammatory; isoandrographolide, 3,19-isopropylideneandrographolide and 14-acetylandrographolide are tumor suppressive; arabinogalactan proteins are anti-hepatotoxic. The four flavonoids from A. paniculata, namely 7-O-methylwogonin, apigenin, onysilin and 3,4-dicaffeoylquinic acid are anti-atherosclerotic.
Andrograpanin, isolated from Andrographis paniculata, exhibits anti-inflammatory property in lipopolysaccharide-induced macrophage cells through down-regulating the p38 MAPKs signaling pathways.[Pubmed:18486905]
Int Immunopharmacol. 2008 Jul;8(7):951-8.
Andrographis paniculata Nees is an official herbal medicine for treatment of infection and inflammation in China. Andrograpanin, the one of diterpene lactones in A. paniculata, is a hydrolysate from neoandrographolide in vivo and in vitro. The goal of the present study was to investigate Andrograpanin which effects on over production of nitric oxide (NO) and pro-inflammatory cytokines (TNF-alpha, IL-6 and IL-12p70) and the key signaling pathways involved in lipopolysaccharide (LPS)-activated macrophage cells. The results showed that NO and all three pro-inflammatory cytokines were inhibited by Andrograpanin (15-90 microM) in a dose-dependent manner. The RT-PCR and western blotting assays showed that Andrograpanin inhibited productions of NO and pro-inflammatory cytokines through down-regulating iNOS and pro-inflammatory cytokines gene expression levels. Further studies suggested that down-regulation of p38 mitogen-activated protein kinase (MAPKs) signaling pathways were involved in the anti-inflammatory activities of Andrograpanin. This study provided evidences that Andrograpanin might be useful as a potential anti-inflammatory leading compound for inflammatory drug development.
Andrograpanin, a compound isolated from anti-inflammatory traditional Chinese medicine Andrographis paniculata, enhances chemokine SDF-1alpha-induced leukocytes chemotaxis.[Pubmed:15937916]
J Cell Biochem. 2005 Aug 1;95(5):970-8.
Andrographis paniculata is a traditional Chinese medicine (TCM) that has been effectively used for treatment of infection, inflammation, cold, fever, and diarrhea in China. However, mechanism of its therapeutic function is not well known. In the current study, we showed one of its components, Andrograpanin, could enhance chemokine stromal cell-derived factor-1alpha (SDF-1alpha) induced chemotaxis in Jurkat and THP-1 cells. Further study demonstrated that this kind of effect was CXC chemokine receptor-4 (CXCR4) specific, since Andrograpanin could not enhance other chemokines, such as RANTES, monocyte chemotactic protein-1 (MCP-1), etc. induced cell chemotaxis. Mechanisms of Andrograpanin exerting its effect were not directly in the receptor and G protein coupling level because it had no effect on the binding of SDF-1 to CXCR4, SDF-1 induced G protein activation and adenyly cyclase inhibition. However, receptor internalization might be involved, since we found it significantly reduced SDF-1alpha-induced CXCR4 internalization.
A new bis-andrographolide ether from Andrographis paniculata nees and evaluation of anti-HIV activity.[Pubmed:15702635]
Nat Prod Res. 2005 Apr;19(3):223-30.
Novel bis-andrographolide ether (1) and six known compounds andrographolide, 14-deoxy-11,12-didehydroandrographolide, Andrograpanin, 14-deoxyandrographolide, (+/-)-5-hydroxy-7,8-dimethoxyflavanone, and 5-hydroxy-7,8-dimethoxyflavone have been isolated from the aerial parts of Andrographis paniculata and their structures were established by spectral data. All the isolates were tested for the anti-HIV and cytotoxic activity.


