Incarvine ACAS# N/A |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | N/A | SDF | File under preparation. |
| PubChem ID | 10414603 | Appearance | Powder |
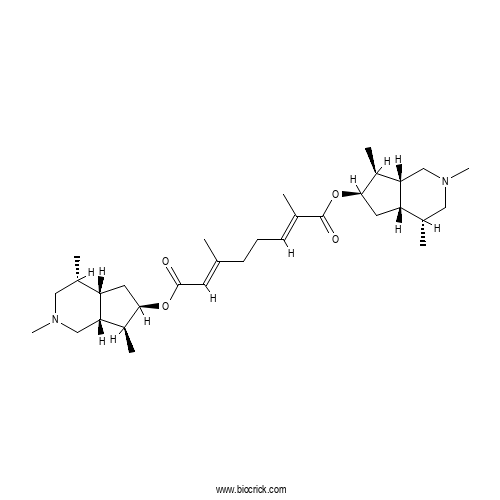
| Formula | C32H52N2O4 | M.Wt | 528.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | bis[(4R,4aS,6R,7S,7aR)-2,4,7-trimethyl-1,3,4,4a,5,6,7,7a-octahydrocyclopenta[c]pyridin-6-yl] (2E,6E)-2,6-dimethylocta-2,6-dienedioate | ||
| SMILES | CC1CN(CC2C1CC(C2C)OC(=O)C=C(C)CCC=C(C)C(=O)OC3CC4C(CN(CC4C3C)C)C)C | ||
| Standard InChIKey | SNACZJFGFSJVFX-OYERZMFVSA-N | ||
| Standard InChI | InChI=1S/C32H52N2O4/c1-19(12-31(35)37-29-13-25-21(3)15-33(7)17-27(25)23(29)5)10-9-11-20(2)32(36)38-30-14-26-22(4)16-34(8)18-28(26)24(30)6/h11-12,21-30H,9-10,13-18H2,1-8H3/b19-12+,20-11+/t21-,22-,23-,24-,25-,26-,27-,28-,29+,30+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Incarvine A Dilution Calculator

Incarvine A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8911 mL | 9.4554 mL | 18.9107 mL | 37.8215 mL | 47.2769 mL |
| 5 mM | 0.3782 mL | 1.8911 mL | 3.7821 mL | 7.5643 mL | 9.4554 mL |
| 10 mM | 0.1891 mL | 0.9455 mL | 1.8911 mL | 3.7821 mL | 4.7277 mL |
| 50 mM | 0.0378 mL | 0.1891 mL | 0.3782 mL | 0.7564 mL | 0.9455 mL |
| 100 mM | 0.0189 mL | 0.0946 mL | 0.1891 mL | 0.3782 mL | 0.4728 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Vatalbinoside F
Catalog No.:BCN0280
CAS No.:
- Vatalbinoside I
Catalog No.:BCN0279
CAS No.:
- Vatalbinoside J
Catalog No.:BCN0278
CAS No.:
- Vatalbinoside H
Catalog No.:BCN0277
CAS No.:
- Vatalbinoside G
Catalog No.:BCN0276
CAS No.:
- Vatalbinoside C
Catalog No.:BCN0275
CAS No.:
- Vatalbinoside A
Catalog No.:BCN0274
CAS No.:
- Soyasaponin A1
Catalog No.:BCN0289
CAS No.:78693-94-4
- Soyasaponin A3
Catalog No.:BCN0288
CAS No.:114077-04-2
- Soyasaponin III
Catalog No.:BCN0287
CAS No.:55304-02-4
- Vaticanol B
Catalog No.:BCN0273
CAS No.:287101-83-1
- Capsiate
Catalog No.:BCN0272
CAS No.:205687-01-0
- Incarvine C
Catalog No.:BCN0282
CAS No.:
- Incarvine D
Catalog No.:BCN0283
CAS No.:
- Incarvine E
Catalog No.:BCN0284
CAS No.:
- Incarvine F
Catalog No.:BCN0285
CAS No.:
- Incarvilone A
Catalog No.:BCN0286
CAS No.:
- Roridin A
Catalog No.:BCN0290
CAS No.:14729-29-4
- Agroclavine
Catalog No.:BCN0291
CAS No.:548-42-5
- Ergocornine
Catalog No.:BCN0292
CAS No.:564-36-3
- Procyanidin A4
Catalog No.:BCN0293
CAS No.:111466-29-6
- L-(±)-Alliin
Catalog No.:BCN0294
CAS No.:17795-26-5
- Andrograpanin
Catalog No.:BCN0295
CAS No.:82209-74-3
- Anemonin
Catalog No.:BCN0296
CAS No.:508-44-1
Structure-antinociceptive activity studies of incarvillateine, a monoterpene alkaloid from Incarvillea sinensis.[Pubmed:11301854]
Planta Med. 2001 Mar;67(2):114-7.
Incarvillateine (1), a new monoterpene alkaloid carrying a characteristic cyclobutane ring, has been found to show significant antinociceptive activity in a formalin-induced pain model in mice. To investigate the correlation between its structure and antinociceptive activity, and especially to study whether a cyclobutane ring is necessary or not for expression of activity, we evaluated the antinociceptive activity of two constructive units of incarvillateine, such as a monoterpene unit (incarvilline, 3) and a phenylpropanoid unit (ferulic acid, 2) in the formalin test, and compared activity of the units with that of incarvillateine. Furthermore, in order to obtain more information about the structure-activity relationships, monoterpene alkaloid derivatives, such as incarvine C (5, a precursor of incarvillateine), Incarvine A (4, an ester compound comprised of two monoterpene alkaloids and a monoterpene) and 3,3'-demethoxy-4,4'-dehydroxyincarvillateine (6, a synthetic new compound), were examined. The antinociceptive effect of 3,3'-demethoxy-4,4'-dehydroxyincarvillateine was equal to that of incarvillateine. Meanwhile, the other compounds exhibited no or weak activity. These results suggested that the cyclobutane moiety of incarvillateine plays an important role in expression of antinociceptive action.


