CapsiateCAS# 205687-01-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 205687-01-0 | SDF | Download SDF |
| PubChem ID | 9839519 | Appearance | Powder |
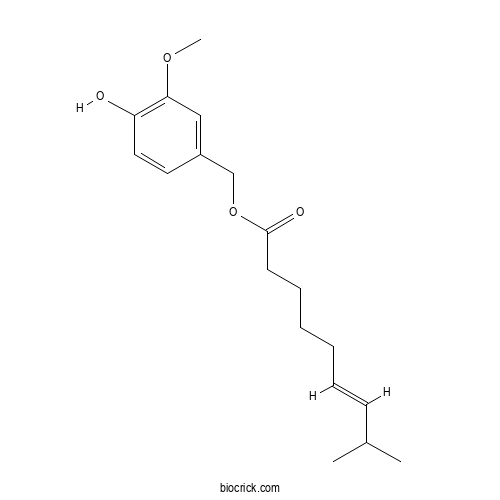
| Formula | C18H26O4 | M.Wt | 306.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4-hydroxy-3-methoxyphenyl)methyl (E)-8-methylnon-6-enoate | ||
| SMILES | CC(C)C=CCCCCC(=O)OCC1=CC(=C(C=C1)O)OC | ||
| Standard InChIKey | ZICNYIDDNJYKCP-SOFGYWHQSA-N | ||
| Standard InChI | InChI=1S/C18H26O4/c1-14(2)8-6-4-5-7-9-18(20)22-13-15-10-11-16(19)17(12-15)21-3/h6,8,10-12,14,19H,4-5,7,9,13H2,1-3H3/b8-6+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Capsiate Dilution Calculator

Capsiate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2637 mL | 16.3185 mL | 32.6371 mL | 65.2742 mL | 81.5927 mL |
| 5 mM | 0.6527 mL | 3.2637 mL | 6.5274 mL | 13.0548 mL | 16.3185 mL |
| 10 mM | 0.3264 mL | 1.6319 mL | 3.2637 mL | 6.5274 mL | 8.1593 mL |
| 50 mM | 0.0653 mL | 0.3264 mL | 0.6527 mL | 1.3055 mL | 1.6319 mL |
| 100 mM | 0.0326 mL | 0.1632 mL | 0.3264 mL | 0.6527 mL | 0.8159 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Colnelenic acid
Catalog No.:BCN0271
CAS No.:52591-16-9
- Nordihydrocapsiate
Catalog No.:BCN0270
CAS No.:220012-53-3
- Dihydrocapsiate
Catalog No.:BCN0269
CAS No.:205687-03-2
- Isovaltrate
Catalog No.:BCN0268
CAS No.:31078-10-1
- Eleutheroside B1
Catalog No.:BCN0267
CAS No.:16845-16-2
- (-)-Conduritol F
Catalog No.:BCN0266
CAS No.:6090-98-8
- Cynandione A
Catalog No.:BCN0265
CAS No.:168706-29-4
- Wilfoside C 1N
Catalog No.:BCN0264
CAS No.:96829-53-7
- Bungeiside B
Catalog No.:BCN0263
CAS No.:149561-88-6
- Wilfoside K 1N
Catalog No.:BCN0262
CAS No.:100802-91-3
- (Z)-5-(Heptadec-10-en-1-yl)resorcinol
Catalog No.:BCN0261
CAS No.:52483-21-3
- Belamcandol B
Catalog No.:BCN0260
CAS No.:137786-94-8
- Vaticanol B
Catalog No.:BCN0273
CAS No.:287101-83-1
- Soyasaponin III
Catalog No.:BCN0287
CAS No.:55304-02-4
- Soyasaponin A3
Catalog No.:BCN0288
CAS No.:114077-04-2
- Soyasaponin A1
Catalog No.:BCN0289
CAS No.:78693-94-4
- Vatalbinoside A
Catalog No.:BCN0274
CAS No.:
- Vatalbinoside C
Catalog No.:BCN0275
CAS No.:
- Vatalbinoside G
Catalog No.:BCN0276
CAS No.:
- Vatalbinoside H
Catalog No.:BCN0277
CAS No.:
- Vatalbinoside J
Catalog No.:BCN0278
CAS No.:
- Vatalbinoside I
Catalog No.:BCN0279
CAS No.:
- Vatalbinoside F
Catalog No.:BCN0280
CAS No.:
- Incarvine A
Catalog No.:BCN0281
CAS No.:
Acute Response to Capsiate Supplementation at Rest and during Exercise on Energy Intake, Appetite, Metabolism, and Autonomic Function: A Randomized Trial.[Pubmed:34252340]
J Am Coll Nutr. 2021 Jul 12:1-10.
OBJECTIVE: The purpose of the present study was to examine the effect of Capsiate supplementation on energy intake, self-reported appetite-related sensations, energy expenditure, fat oxidation, and autonomic parameters with and without an exercise intervention. METHODS: Thirteen healthy men completed four randomized trials: two trials for the control condition (without exercise), one with Capsiate supplementation (CTRLcap) and one with a placebo (CTRLpla), and two trials for the exercise condition, one with Capsiate supplementation (EXcap) and one with placebo (EXpla). Exercise sessions were performed 150 min after the consumption of a standardized breakfast, and supplementation 115 min after consumption of breakfast. An ad libitum buffet was offered 200 min following the completion of the standardized breakfast, and energy intake (EI) and relative energy intake (REI) (relative energy intake = energy intake - energy expenditure related to exercise) were evaluated. RESULTS: There were no significant effects on EI, self-reported appetite sensations, fat oxidation, and energy expenditure. REI was reduced in conditions involving EX when compared to CTRL. A low-frequency to high-frequency ratio for heart rate variability was higher in CTRLcap (1.6 +/- 1.1) vs. CTRLpla (1.2 +/- 0.9) (p = 0.025; d = 0.39). CONCLUSION: Acute Capsiate supplementation combined with aerobic exercise has limited effects on the examined variables (EI, REI, fat oxidation, energy expenditure, and autonomic parameters), while changes in the autonomic nervous system function in the absence of exercise may have occurred without influencing other variables. CLINICAL TRIAL REGISTRATION: ensaiosclinicos.gov.br number, RBR-5pckyr https://ensaiosclinicos.gov.br/rg/RBR-5pckyr.
Chronic capsiate supplementation increases fat-free mass and upper body strength but not the inflammatory response to resistance exercise in young untrained men: a randomized, placebo-controlled and double-blind study.[Pubmed:34154603]
J Int Soc Sports Nutr. 2021 Jun 21;18(1):50.
BACKGROUND: Acute capsaicinoid and capsinoid supplementation has endurance and resistance exercise benefits; however, if these short-term performance benefits translate into chronic benefits when combined with resistance training is currently unknown. This study investigated changes of chronic Capsiate supplementation on muscular adaptations, inflammatory response and performance in untrained men. METHODS: Twenty untrained men were randomized to ingest 12 mg Capsiate (CAP) or placebo in a parallel, double-blind design. Body composition and performance were measured at pre-training and after 6 weeks of resistance training. An acute resistance exercise session test was performed pre and post-intervention. Blood samples were collected at rest and post-resistance exercise to analyze Tumor necrosis factor- (TNF-), Soluble TNF- receptor (sTNF-r), Interleukin-6 (IL-6) and Interleukin-10 (IL-10). RESULTS: Exercise and CAP supplementation increased fat-free mass in comparison to baseline by 1.5 kg (P < 0.001), however, the majority of the increase (1.0 kg) resulted from an increase in total body water. The CAP change scores for fat-free mass were significantly greater in comparison to the placebo (CAP %= 2.1 +/- 1.8 %, PLA %= 0.7 +/- 1.3 %, P = 0.043) and there was a significant difference between groups in the bench press exercise (P = 0.034) with greater upper body strength change score for CAP (%= 13.4 +/- 9.1 %) compared to placebo (%= 5.8 +/- 5.2 %), P = 0.041. CAP had no effect on lower body strength and no supplementation interactions were observed for all cytokines in response to acute resistance exercise (P > 0.05). CONCLUSION: Chronic Capsiate supplementation combined with resistance training during short period (6 weeks) increased fat-free mass and upper body strength but not inflammatory response and performance in young untrained men.
Sweet pepper and its principle constituent capsiate: functional properties and health benefits.[Pubmed:33951968]
Crit Rev Food Sci Nutr. 2021 May 6:1-25.
Capsiate is a non-pungent analogue of capsaicin. It belongs to the family of capsinoids which are esters of vanillyl alcohol with fatty acids while capsaicin belongs to the family of capsaicinoids that are amides of vanillylamine with a variety of branched-chain fatty acids. While capsaicin is extensively reported for plethora of pharmacological actions, Capsiate remains much less explored. Extracted from various species of Capsicum plant, the molecule has also been chemically synthesized via a number of synthetic and enzymatic routes. Based on its action on transient receptor potential vanilloid subfamily member 1 receptors, recent research has focused on its potential roles in treatment of obesity, metabolic disorders, cancer, cardiovascular disorders and gastro-intestinal disorders. Its toxicity profile has been reported to be much safe. The molecule, however, faces the challenge of low aqueous solubility and stability. It has been commercialized for its use as a weight loss supplement. However, the therapeutic potential of the compound which is much beyond boosting metabolism remains unexplored hitherto. This comprehensive review summarizes the studies demonstrating the therapeutic potential of Capsiate in various pathological conditions. Discussed also are potential future directions for formulation strategies to develop efficient, safe and cost-effective dosage forms of Capsiate to explore its role in various disease conditions. The databases investigated include Cochrane library, Medline, Embase, Pubmed and in-house databases. The search terms were "Capsiate," "capsinoids," "thermogenesis," and their combinations. The articles were screened for relevance by going through their abstract. All the articles pertaining to physicochemical, physiological, pharmacological and therapeutic effects of Capsiate have been included in the manuscript.
The gut microbiota metabolite capsiate promotes Gpx4 expression by activating TRPV1 to inhibit intestinal ischemia reperfusion-induced ferroptosis.[Pubmed:33779497]
Gut Microbes. 2021 Jan-Dec;13(1):1-21.
Ferroptosis, a new type of cell death has been found to aggravate intestinal ischemia/reperfusion (I/R) injury. However, little is known about the changes of gut microbiota and metabolites in intestinal I/R and the role of gut microbiota metabolites on ferroptosis-induced intestinal I/R injury. This study aimed to establish a mouse intestinal I/R model and ileum organoid hypoxia/reoxygenation (H/R) model to explore the changes of the gut microbiota and metabolites during intestinal I/R and protective ability of Capsiate (CAT) against ferroptosis-dependent intestinal I/R injury. Intestinal I/R induced disturbance of gut microbiota and significant changes in metabolites. We found that CAT is a metabolite of the gut microbiota and that CAT levels in the preoperative stool of patients undergoing cardiopulmonary bypass were negatively correlated with intestinal I/R injury. Furthermore, CAT reduced ferroptosis-dependent intestinal I/R injury in vivo and in vitro. However, the protective effects of CAT against ferroptosis-dependent intestinal I/R injury were abolished by RSL3, an inhibitor of glutathione peroxidase 4 (Gpx4), which is a negative regulator of ferroptosis. We also found that the ability of CAT to promote Gpx4 expression and inhibit ferroptosis-dependent intestinal I/R injury was abrogated by JNJ-17203212, an antagonist of transient receptor potential cation channel subfamily V member 1 (TRPV1). This study suggests that the gut microbiota metabolite CAT enhances Gpx4 expression and inhibits ferroptosis by activating TRPV1 in intestinal I/R injury, providing a potential avenue for the management of intestinal I/R injury.
Consumption of chilies and sweet peppers is associated with lower risk of sarcopenia in older adults.[Pubmed:33770761]
Aging (Albany NY). 2021 Mar 26;13(6):9135-9142.
BACKGROUND: Sarcopenia is an aging-related loss of muscle mass and function, which induces numerous adverse outcomes. Capsaicin and Capsiate, separately extracted from chilies and sweet peppers, have the potential to induce muscle hypertrophy via activation of transient receptor potential vanilloid 1. The present study aimed to investigate whether chili and sweet pepper consumption are related to sarcopenia in the elderly general population. METHODS: A cross-sectional study with 2,451 participants was performed. Dietary chili and sweet pepper consumption were assessed using a validated self-administered food frequency questionnaire. Sarcopenia was defined according to the consensus of the Asian Working Group for Sarcopenia. Logistic regressions were performed to measure the effect of chili and sweet pepper consumption on sarcopenia. RESULTS: The prevalence of sarcopenia was 16.1%. After adjustment for potential confounding variables, the odds ratios (95% confidence intervals) for sarcopenia across chili and sweet pepper consumption categories were 1.00 (reference) for almost never, 0.73 (0.55, 0.97) and 0.73 (0.56, 0.96) for /=2-3 times/week (both P for trend <0.01), respectively. CONCLUSION: The present study showed that higher consumption of chilies and sweet peppers was related to a lower risk of sarcopenia in older adults.
Capsiate Intake with Exercise Training Additively Reduces Fat Deposition in Mice on a High-Fat Diet, but Not without Exercise Training.[Pubmed:33466647]
Int J Mol Sci. 2021 Jan 14;22(2). pii: ijms22020769.
While exercise training (ET) is an efficient strategy to manage obesity, it is recommended with a dietary plan to maximize the antiobesity functions owing to a compensational increase in energy intake. Capsiate is a notable bioactive compound for managing obesity owing to its capacity to increase energy expenditure. We aimed to examine whether the antiobesity effects of ET can be further enhanced by Capsiate intake (CI) and determine its effects on resting energy expenditure and metabolic molecules. Mice were randomly divided into four groups (n = 8 per group) and fed high-fat diet. Mild-intensity treadmill ET was conducted five times/week; Capsiate (10 mg/kg) was orally administered daily. After 8 weeks, resting metabolic rate and metabolic molecules were analyzed. ET with CI additively reduced the abdominal fat rate by 18% and solely upregulated beta-3-adrenoceptors in adipose tissue (p = 0.013) but did not affect the metabolic molecules in skeletal muscles. Surprisingly, CI without ET significantly increased the abdominal fat rate (p = 0.001) and reduced energy expenditure by 9%. Therefore, Capsiate could be a candidate compound for maximizing the antiobesity effects of ET by upregulating beta-3-adrenoceptors in adipose tissue, but CI without ET may not be beneficial in managing obesity.
Capsaicin Analogue Supplementation Does Not Improve 10 km Running Time-Trial Performance in Male Amateur Athletes: A Randomized, Crossover, Double-Blind and Placebo-Controlled Study.[Pubmed:33374147]
Nutrients. 2020 Dec 24;13(1). pii: nu13010034.
BACKGROUND: To investigate the acute effects of a capsaicin analogue supplement on 10 km time-trial performance and physiological responses in amateur athletes. METHODS: Twenty-one participants (age = 29.3 +/- 5.5 years, weight 74.2 +/- 11.3 kg, height 176.0 +/- 0.0 cm, fat mass 12.7 +/- 3.8%, V O2max 62.7 +/- 8.4 mL.k(-1).min(-1)), completed two randomized, double-blind trials: capsaicin analogue condition (Capsiate (CAP) = 24 mg) or a placebo (PLA) condition. The participants consumed two doses of 12 mg of CAP or PLA capsule 45 min before and immediately at the start of each trial. The time required to complete 10 km, lactate concentration, maximum heart rate (HRpeak), and rating of perceived exertion (RPE) were recorded. RESULTS: The 10 km time-trial performance (CAP = 45.07 +/- 6.41 min vs. PLA = 45.13 +/- 6.73, p = 0.828) was not statistically significantly different between conditions. No statistically significant differences between conditions were detected for lactate concentration (p = 0.507), HRpeak (p = 0.897) and RPE (p = 0.517). CONCLUSION: Two doses of a 12 mg Capsaicin analogue supplement did not improve performance and physiological responses in a 10 km running time-trial in amateur athletes.
Effect of mild-intensity exercise training with capsiate intake on fat deposition and substrate utilization during exercise in diet-induced obese mice.[Pubmed:33108711]
Phys Act Nutr. 2020 Sep;24(3):1-6.
Purpose: While the anti-obesity effects of exercise and Capsiate are well-observed individually, the effect of exercise with Capsiate intake has not been systematically explored yet. Therefore, the purpose of this study is to investigate whether the anti-obesity effects of exercise training can be further enhanced by Capsiate intake. Methods: 8-week-old male mice were divided into 3 groups (n = 8 per group): sedentary group (SED; nontrained), exercise-trained group (EXE) and exercisetrained group with 10 mg/kg of Capsiate intake (EXE+CAP). All mice were offered high-fat diet and water ad libitum. The mild-intensity treadmill training was conducted 5 times a week for 8 weeks. After 8 weeks, metabolism during exercise and abdominal fat weight were measured. Results: Body weight and the rate of total abdominal fat were significantly less in EXE+CAP than in SED but not between EXE and SED. The average of respiratory exchange rate during exercise was significantly much lower in EXE+SED (p = 0.003) compared to the difference between EXE and SED (p = 0.025). Likewise, the fat oxidation during exercise was significantly much higher in EXE+SED (p = 0.016) compared to the difference between EXE and SED (p = 0.045). Then, the carbohydrate oxidation during exercise was significantly much lower in EXE+SED (p = 0.003) compared to the difference between EXE and SED (p = 0.028). Conclusion: In conclusion, the anti-obesity functions of exercise training can be further enhanced by Capsiate intake by increasing fat oxidation during exercise. Therefore, we suggest that Capsiate could be a candidate supplement which can additively ameliorate obesity when combined with exercise.
Content of Capsaicinoids and Capsiate in "Filius" Pepper Varieties as Affected by Ripening.[Pubmed:32957596]
Plants (Basel). 2020 Sep 17;9(9). pii: plants9091222.
Peppers are fruits with wide genetic variability and multiple ways of being consumed that hold a relevant position in the human diet. Nowadays, consumers are interested in new gastronomic experiences provided by pepper cultivars that present new shapes, colors, and flavors while preserving their bioactive compounds, such as their capsaicinoids and capsinoids. However, numerous changes take place during their development that may alter their biological properties. Therefore, this work evaluates the capsaicinoid and Capsiate contents in two traditional varieties of ornamental peppers ("Filius Blue" and "Filius Green'") during fruit maturation. The aim is to determine the ideal harvesting moment depending on the farmer's objective (e.g., achieving a specific color, shape, or flavor; achieving the maximum concentrations of bioactive compounds). The capsaicinoid contents followed different patterns in the two varieties analyzed. The "Filius Blue" variety exhibited increasing concentrations of capsaicinoids up to the 41st day post-anthesis (dpa), from which point on this trend was reversed. The concentrations in the "Filius Green" variety increased and decreased several times, reaching maximum concentrations on the 69th dpa. Regarding Capsiate contents, both varieties varied in the same way, reaching maximum concentrations on the 34th dpa and then decreasing.
Acute Capsaicin Analog Supplementation Improves 400 M and 3000 M Running Time-Trial Performance.[Pubmed:32509117]
Int J Exerc Sci. 2020 May 1;13(2):755-765. eCollection 2020.
Objectives: Performance in running-based sport depends on the ability to perform repetitive high intensity muscle contractions. Previous studies have shown that capsaicin analog (CAP) (i.e. Capsiate) supplementation may improve this performance. The purpose of this study was to investigate the acute effect of CAP supplementation on short (400 m) and middle distance (3000 m) running time-trial performance, maximum heart rate (HR), and rate of perceived exertion (RPE). Methods: Twelve physically active men completed four randomized, double-blind trials: CAP condition (12 mg) or a placebo condition. Forty-five minutes after supplementation, the participants performed a 400- or 3000-meter running time trial. Time (in seconds) was recorded. HR was analyzed at rest and immediately post-exercise, and RPE was collected immediately after exercise. Results: For both the 400 m time-trial (CAP = 66.4 + 4.2 sec vs Placebo = 67.1 + 4.8 sec, p = 0.046) and the 3000 m time-trial (CAP = 893.9 +/- 46.8 sec vs Placebo = 915.2 +/- 67.6 sec, p = 0.015), the time in seconds was significantly less in the CAP compared to placebo conditions. There were no statistically significant differences for HR and RPE in any condition. Conclusion: In summary, acute CAP supplementation improved 400 m and 3000 m running time-trial performance in a distance-dependent way but without modifying the HR and RPE.
Assessment of Capsaicinoid and Capsinoid Accumulation Patterns during Fruit Development in Three Chili Pepper Genotypes (Capsicum spp.) Carrying Pun1 and pAMT Alleles Related to Pungency.[Pubmed:31613626]
J Agric Food Chem. 2019 Nov 6;67(44):12219-12227.
Quantification, using an accurate analytical approach, of capsinoids and capsaicinoids was performed on three chili pepper (Capsicum spp.) genotypes: "Chiltepin", "Tampiqueno 74", and "Bhut Jolokia" at various stages of fruit development. The accumulation of capsinoids, in all these peppers started between 10 to 20 days post-anthesis (dpa), increased and reached the highest capsinoid amount at 40 dpa, and then decreased until 60 dpa. Conversely, capsaicinoids could already be determined at 10 dpa in "Bhut Jolokia" and their accumulation pattern was different from that of the capsinoids in this genotype. The Capsiate/dihydroCapsiate ratio presented a higher variation between genotypes and developmental stages than the capsaicin/dihydrocapsaicin ratio. Capsinoid ratios (4-24%) and Pun1/pAMT genotyping were determined. These results provide information on the progress of the accumulation of capsinoids in the aforementioned pungent and superhot cultivars and could support future breeding studies toward the understanding of the factors affecting their accumulation.
Biological Evaluation of Natural and Synthesized Homovanillic Acid Esters as Inhibitors of Intestinal Fatty Acid Uptake in Differentiated Caco-2 Cells.[Pubmed:31591297]
Molecules. 2019 Oct 7;24(19). pii: molecules24193599.
With raising prevalence of obesity, the regulation of human body fat is increasingly relevant. The modulation of fatty acid uptake by enterocytes represents a promising target for body weight maintenance. Recent results demonstrated that the trigeminal active compounds capsaicin, nonivamide, and trans-pellitorine dose-dependently reduce fatty acid uptake in differentiated Caco-2 cells as a model for the intestinal barrier. However, non-pungent alternatives have not been investigated and structural determinants for the modulation of intestinal fatty acid uptake have not been identified so far. Thus, based on the previous results, we synthesized 23 homovanillic acid esters in addition to the naturally occurring Capsiate and screened them for their potential to reduce intestinal fatty acid uptake using the fluorescent fatty acid analog Bodipy-C12 in differentiated Caco2 cells as an enterocyte model. Whereas pre-incubation with 100 microM Capsiate did not change fatty acid uptake by Caco-2 enterocytes, a maximum inhibition of -47% was reached using 100 microM 1methylpentyl-2-(4-hydroxy-3-methoxy-phenyl)acetate. Structural analysis of the 24 structural analogues tested in the present study revealed that a branched fatty acid side chain, independent of the chain length, is one of the most important structural motifs associated with inhibition of fatty acid uptake in Caco-2 enterocytes. The results of the present study may serve as an important basis for designing potent dietary inhibitors of fatty acid uptake.


