Eleutheroside B1CAS# 16845-16-2 |
- Calycanthoside
Catalog No.:BCN5580
CAS No.:483-91-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 16845-16-2 | SDF | Download SDF |
| PubChem ID | 12302276 | Appearance | Powder |
| Formula | C17H20O10 | M.Wt | 384.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
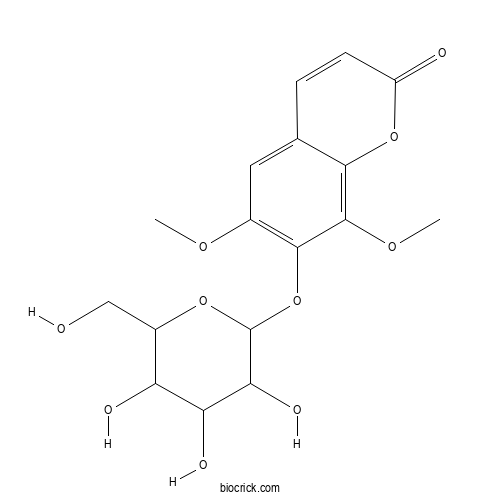
| Chemical Name | 6,8-dimethoxy-7-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-2-one | ||
| SMILES | COC1=C(C(=C2C(=C1)C=CC(=O)O2)OC)OC3C(C(C(C(O3)CO)O)O)O | ||
| Standard InChIKey | IKUQEFGEUOOPGY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H20O10/c1-23-8-5-7-3-4-10(19)26-14(7)16(24-2)15(8)27-17-13(22)12(21)11(20)9(6-18)25-17/h3-5,9,11-13,17-18,20-22H,6H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Eleutheroside B1 Dilution Calculator

Eleutheroside B1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6021 mL | 13.0107 mL | 26.0213 mL | 52.0427 mL | 65.0533 mL |
| 5 mM | 0.5204 mL | 2.6021 mL | 5.2043 mL | 10.4085 mL | 13.0107 mL |
| 10 mM | 0.2602 mL | 1.3011 mL | 2.6021 mL | 5.2043 mL | 6.5053 mL |
| 50 mM | 0.052 mL | 0.2602 mL | 0.5204 mL | 1.0409 mL | 1.3011 mL |
| 100 mM | 0.026 mL | 0.1301 mL | 0.2602 mL | 0.5204 mL | 0.6505 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (-)-Conduritol F
Catalog No.:BCN0266
CAS No.:6090-98-8
- Cynandione A
Catalog No.:BCN0265
CAS No.:168706-29-4
- Wilfoside C 1N
Catalog No.:BCN0264
CAS No.:96829-53-7
- Bungeiside B
Catalog No.:BCN0263
CAS No.:149561-88-6
- Wilfoside K 1N
Catalog No.:BCN0262
CAS No.:100802-91-3
- (Z)-5-(Heptadec-10-en-1-yl)resorcinol
Catalog No.:BCN0261
CAS No.:52483-21-3
- Belamcandol B
Catalog No.:BCN0260
CAS No.:137786-94-8
- Deoxy euphorbia factor L1
Catalog No.:BCN0259
CAS No.:247099-01-0
- Euphorbia factor L22
Catalog No.:BCN0258
CAS No.:1613700-09-6
- Planispine A
Catalog No.:BCN0257
CAS No.:1202761-42-9
- Secotubeimoside I
Catalog No.:BCN0256
CAS No.:106235-32-9
- Fuzitine
Catalog No.:BCN0255
CAS No.:142287-96-5
- Isovaltrate
Catalog No.:BCN0268
CAS No.:31078-10-1
- Dihydrocapsiate
Catalog No.:BCN0269
CAS No.:205687-03-2
- Nordihydrocapsiate
Catalog No.:BCN0270
CAS No.:220012-53-3
- Colnelenic acid
Catalog No.:BCN0271
CAS No.:52591-16-9
- Capsiate
Catalog No.:BCN0272
CAS No.:205687-01-0
- Vaticanol B
Catalog No.:BCN0273
CAS No.:287101-83-1
- Soyasaponin III
Catalog No.:BCN0287
CAS No.:55304-02-4
- Soyasaponin A3
Catalog No.:BCN0288
CAS No.:114077-04-2
- Soyasaponin A1
Catalog No.:BCN0289
CAS No.:78693-94-4
- Vatalbinoside A
Catalog No.:BCN0274
CAS No.:
- Vatalbinoside C
Catalog No.:BCN0275
CAS No.:
- Vatalbinoside G
Catalog No.:BCN0276
CAS No.:
Effect of eleutheroside B1 on noncoding RNAs and protein profiles of influenza A virusinfected A549 cells.[Pubmed:31985023]
Int J Mol Med. 2020 Mar;45(3):753-768.
Influenza viruses often pose a serious threat to animals and human health. In an attempt to explore the potential of herbal medicine as a treatment for influenza virus infection, Eleutheroside B1, a coumarin compound extracted from herba sarcandrae, was identified, which exhibited antiviral and antiinflammatory activities against influenza A virus. In this study, highthroughput RNA sequencing and isobaric tags for relative and absolute quantification (iTRAQ) assays were performed to determine alterations in the noncoding RNA (ncRNA) transcriptome and proteomics. Bioinformatics and target prediction analyses were used to decipher the potential roles of altered ncRNAs in the function of Eleutheroside B1. Furthermore, long ncRNA (lncRNA) and mRNA coexpressing networks were constructed to analyze the biological functions by Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses. The analysis of RNA sequencing data revealed that 5 differentially expressed ncRNAs were upregulated and 3 ncRNAs were downregulated in the A549 cells infected with A/PR8/34/H1N1, with or without Eleutheroside B1 treatment (PR8+eleu and PR8, respectively). Nuclear paraspeckle assembly transcript 1 (NEAT1) was differentially expressed between the PR8 and A549 cell groups. GO and KEGG pathway analyses indicated that Eleutheroside B1 took advantage of the host cell biological processes and molecular function for its antiviral and antiinflammatory activities, as well as for regulating cytokinecytokine receptor interaction in the immune system, consistent with previous findings. The results of the iTRAQ assays indicated that L antigen family member 3 (LAGE3) protein, essential for tRNA processing, tRNA metabolic processes and ncRNA processing, was downregulated in the PR8+eleu compared with the PR8 group. In the present study, these comprehensive, largescale data analysis enhanced the understanding of multiple aspects of the transcriptome and proteomics that are involved in the antiviral and antiinflammatory activities of Eleutheroside B1. These findings demonstrate the potential of Eleutheroside B1 for use in the prevention and treatment of influenza A virusmediated infections.
Eleutheroside B1 mediates its anti-influenza activity through POLR2A and N-glycosylation.[Pubmed:30226535]
Int J Mol Med. 2018 Nov;42(5):2776-2792.
Influenza viruses represent a serious threat to human health. Although our research group has previously demonstrated the antiviral and antiinflammatory activities of Eleutheroside B1, a detailed explanation of the mechanism by which it is effective against the influenza virus remains to be elucidated. In the present study, the transcriptomic responses of influenza A virusinfected lung epithelial cells (A549) treated with Eleutheroside B1 were investigated using highthroughput RNA sequencing, and potential targets were identified using a molecular docking technique, reverse transcriptionquantitative polymerase chain reaction (RTqPCR) assay, and DNA methylation analysis. The transcriptomic data revealed that there are 1,871 differentially expressed genes (DEGs) between the cells infected with the influenza virus strain variant PR8, and the cells infected with PR8 and treated with Eleutheroside B1. Among the DEGs, RNA polymerase II subunit A (POLR2A; encoding the largest subunit of RNA polymerase II) and mannosidase alpha class II member 1 (MAN2A1) were selected from the molecular docking analysis with Eleutheroside B1. The docking score of Drosophila melanogaster MAN2A1 (3BVT) was 11.3029, whereas that of POLR2A was 9.0133. The RTqPCR results demonstrated that the expression levels of host genes (MAN2A2, POLR2A) and viral genes (PA, PB1, PB2, HA) were downregulated following Eleutheroside B1 treatment. Bisulfitesequencing PCR was performed to investigate whether Eleutheroside B1 was able to modify the DNA methylation of POLR2A, and the results suggested that the average proportion of methylated CpGs (22272 bp) increased significantly following treatment with Eleutheroside B1. Taken together, these findings suggested that Eleutheroside B1 may affect Nglycan biosynthesis, the chemokine signaling pathway, cytokinecytokine receptor interaction and, in particular, may target the POLR2A to inhibit the production of influenza virus genes.
Inhibition viral RNP and anti-inflammatory activity of coumarins against influenza virus.[Pubmed:28081470]
Biomed Pharmacother. 2017 Mar;87:583-588.
Influenza viruses pose a severe threat to human health and a significant increase in antiviral drug-resistant among influenza viruses worldwide has been observed. Therefore, there is an urgent need to develop the new antiviral drugs, specifically from the natural products. In this study, the anti-viral and anti-inflammatory activities of coumarins against influenza A virus in vitro were investigated. One of the derivatives Eleutheroside B1 showed a wide spectrum of anti- human influenza virus effect with the IC50 value of 64-125mug/ml in vitro, but it showed no effects against avian influenza virus. The time of addition was done and the results indicated that it had a potent antiviral effect when added at 0-6h, and also the virus yield was reduced by 60%. The influenza virus ribonucleoprotein was inhibited at 200mug/ml, and also the NP mRNA expression was inhibited at 50 and 200mug/ml. The expression level of cytokines and chemokines influenced by Eleutheroside B1 was further demonstrated, the IL-6, CXCL-8, CCL-2 expression were all inhibited by the eleuthe roside B1 at concentration 200mug/ml. The findings of study suggest that Eleutheroside B1 can be as potential agent to develop for the prevention and treatment of influenza A virus.


