NordihydrocapsiateCAS# 220012-53-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 220012-53-3 | SDF | Download SDF |
| PubChem ID | 9817607 | Appearance | Powder |
| Formula | C17H26O4 | M.Wt | 294.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
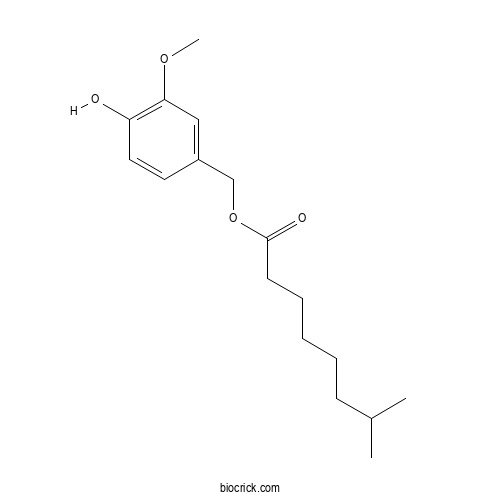
| Chemical Name | (4-hydroxy-3-methoxyphenyl)methyl 7-methyloctanoate | ||
| SMILES | CC(C)CCCCCC(=O)OCC1=CC(=C(C=C1)O)OC | ||
| Standard InChIKey | BXBVPYSHEOQGHP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H26O4/c1-13(2)7-5-4-6-8-17(19)21-12-14-9-10-15(18)16(11-14)20-3/h9-11,13,18H,4-8,12H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Nordihydrocapsiate Dilution Calculator

Nordihydrocapsiate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3967 mL | 16.9837 mL | 33.9674 mL | 67.9348 mL | 84.9185 mL |
| 5 mM | 0.6793 mL | 3.3967 mL | 6.7935 mL | 13.587 mL | 16.9837 mL |
| 10 mM | 0.3397 mL | 1.6984 mL | 3.3967 mL | 6.7935 mL | 8.4918 mL |
| 50 mM | 0.0679 mL | 0.3397 mL | 0.6793 mL | 1.3587 mL | 1.6984 mL |
| 100 mM | 0.034 mL | 0.1698 mL | 0.3397 mL | 0.6793 mL | 0.8492 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dihydrocapsiate
Catalog No.:BCN0269
CAS No.:205687-03-2
- Isovaltrate
Catalog No.:BCN0268
CAS No.:31078-10-1
- Eleutheroside B1
Catalog No.:BCN0267
CAS No.:16845-16-2
- (-)-Conduritol F
Catalog No.:BCN0266
CAS No.:6090-98-8
- Cynandione A
Catalog No.:BCN0265
CAS No.:168706-29-4
- Wilfoside C 1N
Catalog No.:BCN0264
CAS No.:96829-53-7
- Bungeiside B
Catalog No.:BCN0263
CAS No.:149561-88-6
- Wilfoside K 1N
Catalog No.:BCN0262
CAS No.:100802-91-3
- (Z)-5-(Heptadec-10-en-1-yl)resorcinol
Catalog No.:BCN0261
CAS No.:52483-21-3
- Belamcandol B
Catalog No.:BCN0260
CAS No.:137786-94-8
- Deoxy euphorbia factor L1
Catalog No.:BCN0259
CAS No.:247099-01-0
- Euphorbia factor L22
Catalog No.:BCN0258
CAS No.:1613700-09-6
- Colnelenic acid
Catalog No.:BCN0271
CAS No.:52591-16-9
- Capsiate
Catalog No.:BCN0272
CAS No.:205687-01-0
- Vaticanol B
Catalog No.:BCN0273
CAS No.:287101-83-1
- Soyasaponin III
Catalog No.:BCN0287
CAS No.:55304-02-4
- Soyasaponin A3
Catalog No.:BCN0288
CAS No.:114077-04-2
- Soyasaponin A1
Catalog No.:BCN0289
CAS No.:78693-94-4
- Vatalbinoside A
Catalog No.:BCN0274
CAS No.:
- Vatalbinoside C
Catalog No.:BCN0275
CAS No.:
- Vatalbinoside G
Catalog No.:BCN0276
CAS No.:
- Vatalbinoside H
Catalog No.:BCN0277
CAS No.:
- Vatalbinoside J
Catalog No.:BCN0278
CAS No.:
- Vatalbinoside I
Catalog No.:BCN0279
CAS No.:
Activation of transient receptor potential A1 by a non-pungent capsaicin-like compound, capsiate.[Pubmed:21883144]
Br J Pharmacol. 2012 Mar;165(5):1476-86.
BACKGROUND AND PURPOSE: Capsiate is produced by 'CH-19 Sweet' (Capsicum annuun L.), a non-pungent cultivar of red pepper. Like capsaicin, capsiate is thought to enhance energy metabolism by activating the sympathetic nervous system and suppressing inflammation, but the underlying mechanisms for this are uncertain. We previously reported that capsiate could activate transient receptor potential vanilloid 1 (TRPV1), a capsaicin receptor. The purpose of the present study is to investigate whether capsinoids activate other TRP channels. EXPERIMENTAL APPROACH: Using Ca(2+) imaging and whole-cell patch-clamp methods, we analysed the response of TRP channels to three kinds of capsinoids, capsiate, dihydrocapsiate and Nordihydrocapsiate, in HEK293T cells expressing TRP channels or in primary cultures of mouse dorsal root ganglion neurons. KEY RESULTS: We found that in both cell types TRP ankyrin 1 (TRPA1) had a slightly weaker response to capsinoids compared with TRPV1, with the capsiate EC(50) for TRPA1 activation being more than that for TRPV1 activation, and that the capsinoid-evoked action was blocked by a specific TRPA1 antagonist. TRPA1 was activated by capsinoids, but not by their degradation products. Amino acids known to participate in TRPA1 activation following cysteine covalent modification or zinc treatment were not involved in the activation of TRPA1 by capsinoid. CONCLUSIONS AND IMPLICATIONS: Taken together, these results indicate that capsinoids activate TRPA1 by an as yet unknown mechanism, and TRPA1 could be involved in physiological phenomena associated with capsinoid treatment.
Recent advances in the study on capsaicinoids and capsinoids.[Pubmed:20946891]
Eur J Pharmacol. 2011 Jan 10;650(1):1-7.
Chili peppers are the major source of nature capsaicinoids, which consist of capsaicin, dihydrocapsaicin, nordihydrocapsaicin, homodihydrocapsaicin, and homocapsaicin, etc. Capsaicinoids are found to exert multiple pharmacological and physiological effects including the activities of analgesia, anticancer, anti-inflammation, antioxidant and anti-obesity. Therefore, capsaicinoids may have the potential value in clinic for pain relief, cancer prevention and weight loss. In addition, capsaicinoids also display the benefits on cardiovascular and gastrointestinal system. It has been shown that capsaicinoids are potential agonists of capsaicin receptor or transient receptor potential vanilloid subfamily member 1 (TRPV1). They could exert the effects not only through the receptor-dependent pathway but also through the receptor-independent one. CH-19 Sweet peppers are the source of nature capsinoids, which share similar structure with capsaicinoids and consist of capsiate, dihydrocapsiate, and Nordihydrocapsiate, etc, Comparing with capsaicinoids, capsinoids are less pungent and easily broken down in the normal aqueous conditions. So far, it has been found that capsinoids possess the biological properties of antitumor, antioxidant and anti-obesity. Since capsinoids are less toxic than capsaicinoids, therefore, capsinoids may have the advantages over capsaicinoids in clinical applications such as cancer prevention and weight loss.
Studies of the toxicological potential of capsinoids, XIII: inhibitory effects of capsaicin and capsinoids on cytochrome P450 3A4 in human liver microsomes.[Pubmed:20388821]
Int J Toxicol. 2010 Mar;29(2 Suppl):22S-6S.
This study evaluated potential effects of a number of capsinoids (ie, capsiate, dihydrocapsiate, Nordihydrocapsiate) and a single capsaicinoid (ie, capsaicin) on liver microsomal cytochrome P450 3A4-mediated midazolam 1'-hydroxylase activity. Where possible, an inhibition curve was prepared; the concentration at which enzyme activity dropped to 50% was calculated. Capsaicin clearly inhibited cytochrome P450 3A4 activity, losing 50% of the activity at 21.5 micromol/L. No enzyme inhibition was observed in the presence of capsiate, dihydrocapsiate, or Nordihydrocapsiate (<100 micromol/L). Preincubation increased the capsaicin inhibitory activity against cytochrome P450 3A4 in a time-dependent manner. Enzyme activity was slightly reduced by capsiate, dihydrocapsiate, and Nordihydrocapsiate to the same level as that attained with tolbutamide, the negative control compound. Capsaicin was shown to inhibit cytochrome P450 3A4, probably through a mechanism-based inhibition. In contrast, capsiate, dihydrocapsiate, and Nordihydrocapsiate did not inhibit cytochrome P450 3A4 activity and were unlikely to be mechanism-based inhibitors of CYP3A4.
Studies of the toxicological potential of capsinoids, XII: pharmacokinetic study of capsinoid-containing CH-19 Sweet extract in rats.[Pubmed:20388820]
Int J Toxicol. 2010 Mar;29(2 Suppl):15S-21S.
Pharmacokinetics of the main capsinoid components of CH-19 Sweet extract (capsiate, dihydrocapsiate, and Nordihydrocapsiate) were investigated in rats receiving a single gavage dose of extract containing 10 or 100 mg of capsinoids per kilogram in medium-chain triglyceride. Resultant blood levels of these capsinoids and a capsinoid metabolite, vanillyl alcohol, were measured in portal vein and systemic blood. Capsinoids were never detected. Portal compartment vanillyl alcohol concentrations and area under the plasma concentration versus time curve increased approximately with dose, whereas the time to maximum concentration of vanillyl alcohol was independent of dose (30 minutes post dosing), suggesting that precipitation in the stomach or intestines was unlikely. Vanillyl alcohol levels were just barely detectable in systemic plasma (5 minutes post dosing). Significant levels of vanillyl alcohol conjugates, sulfate, and glucuronide were detected in the systemic blood. Given that the orally administered capsinoids were never detected in the portal vein or systemic circulation, these compounds must be broken down (chemically or enzymatically) to vanillyl alcohol.
Assessment of the biological similarity of three capsaicin analogs (Capsinoids) found in non-pungent chili pepper (CH-19 Sweet) fruits.[Pubmed:20139632]
Biosci Biotechnol Biochem. 2010;74(2):274-8.
CH-19 Sweet is a newly found chili pepper breed bearing much less pungent fruits. Because CH-19 Sweet fruits were found to contain three analogs (capsinoids) of capsaicin, a major component of pungency of hot peppers (the analogs are capsiate or CST, dihydrocapsiate or DCT, and Nordihydrocapsiate or NDCT), we assessed in this study the bio-potencies of these three capsinoids by comparing them with capsaicin. The three capsinoids bound to transient potential vanilloid 1 (TRPV1) receptors expressed in cultured cells and activated Ca(2+) influx in a concentration-dependent manner with similar magnitudes. In contrast to capsaicin, capsinoids at the same concentration induced virtually no nociceptive responses when applied to the eyes or the oral cavities of mice. Intravenous administration of capsaicin or 20-fold increased doses of each capsinoid to rats induced significant increases in plasma catecholamine levels. Orally administered, each capsinoid enhanced oxygen consumption in mice. Based on the present results, capsaicin and these three capsinoids should have similar bio-potency, though capsinoids do not generate pungency or sensory irritation.
Effect of topical application of capsaicin and its related compounds on dermal insulin-like growth factor-I levels in mice and on facial skin elasticity in humans.[Pubmed:17307377]
Growth Horm IGF Res. 2007 Apr;17(2):171-6.
Capsaicin increases calcitonin gene-related peptide (CGRP) release from sensory neurons by stimulating vanilloid receptor-1 (VR-1). Since CGRP increases production of insulin-like growth factor-I (IGF-I) in fetal osteoblasts in vitro, it is possible that sensory neuron activation by capsaicin increases production of IGF-I. In the present study, we attempted to determine whether topical application of capsaicin and related compounds increases dermal IGF-I level in mice and whether it increases facial skin elasticity in humans. Topical application of 0.01% capsaicin significantly increased dermal IGF-I levels from 30 to 180min (p<0.01), but not at 360min, after application in mice. Topical application of 0.01% capsaicinoids (dihydrocapsaicin and nordihydrocapsaicin), 0.01% capsinoids (capsiate, dihydrocapsiate and Nordihydrocapsiate), 0.01% anandamide (an endogenous agonist of VR-1), and 0.01% nonylic acid vanillylamide (a synthetic capsaicin) significantly increased dermal IGF-I levels at 30min after topical application in mice (p<0.01). Topical application of 0.01% capsaicin to faces of 17 healthy female volunteers for seven days significantly increased cheek skin elasticity (p<0.01). These observations suggest that topical application of capsaicin and related compounds might be useful in the treatment of detrimental morphological changes of the skin in patients with growth hormone deficiency and those in the elderly by increasing dermal IGF-I levels.
Immunosuppressive activity of capsaicinoids: capsiate derived from sweet peppers inhibits NF-kappaB activation and is a potent antiinflammatory compound in vivo.[Pubmed:12115659]
Eur J Immunol. 2002 Jun;32(6):1753-63.
Capsiate and its dihydroderivatives are the major capsaicinoids of sweet pepper. These new capsaicinoids do not activate the vanilloid receptor type 1 (VR1) but they share with capsaicin (CPS)some biological activities mediated in a VR1-independent fashion. In this study we show that CPS and Nordihydrocapsiate (CPT) inhibit early and late events in T cell activation, including CD69, CD25 and ICAM-1 cell surface expression, progression to the S phase of the cell cycle and proliferation in response to TCR and CD28 co-engagement. Moreover, both CPS and CPT inhibit NF-kappaB activation in response to different agents including TNF-alpha. CPS itself does not affect the DNA-binding ability of NF-kappaB but it prevents IkappaB kinase activation and IkappaBalpha degradation in a dose-dependent manner, without inhibiting the activation of the mitogen-activated protein kinases, p38, extracellular regulated kinase and c-Jun N-terminal protein kinase. Moreover, intraperitoneal pretreatment with CPT prevented mice from lethal septic shock induced by lipopolysaccharide. In a second model of inflammation CPT pretreatment greatly reduced the extensive damage in the glandular epithelium observed in the bowel of DSS-treated mice. Taken together, these results suggest that CPT and related synthetic analogues target specific pathways involved in inflammation, and hold considerable potential for dietary health benefits as well as for pharmaceutical development.
Nordihydrocapsiate, a new capsinoid from the fruits of a nonpungent pepper, capsicum annuum[Pubmed:10075779]
J Nat Prod. 1999 Feb;62(2):335-6.
A new capsiate-like substance, named Nordihydrocapsiate (1), has been isolated from the fruits of a nonpungent cultivar, CH-19 Sweet, of pepper (Capsicum annuum). The structure of 1 was determined to be 4-hydroxy-3-methoxybenzyl 7-methyloctanoate by spectroscopic methods.


