IsovaltrateCAS# 31078-10-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 31078-10-1 | SDF | Download SDF |
| PubChem ID | 92275 | Appearance | Powder |
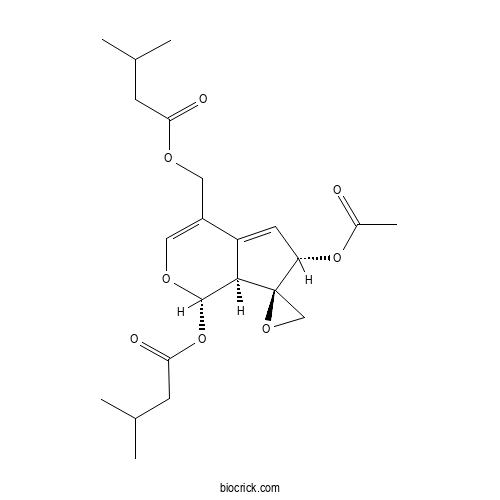
| Formula | C22H30O8 | M.Wt | 422.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1S,6S,7R,7aS)-6-acetyloxy-1-(3-methylbutanoyloxy)spiro[6,7a-dihydro-1H-cyclopenta[c]pyran-7,2'-oxirane]-4-yl]methyl 3-methylbutanoate | ||
| SMILES | CC(C)CC(=O)OCC1=COC(C2C1=CC(C23CO3)OC(=O)C)OC(=O)CC(C)C | ||
| Standard InChIKey | XLACUABANMZLCJ-KVJIRVJXSA-N | ||
| Standard InChI | InChI=1S/C22H30O8/c1-12(2)6-18(24)26-9-15-10-27-21(30-19(25)7-13(3)4)20-16(15)8-17(29-14(5)23)22(20)11-28-22/h8,10,12-13,17,20-21H,6-7,9,11H2,1-5H3/t17-,20+,21-,22+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isovaltrate Dilution Calculator

Isovaltrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3669 mL | 11.8343 mL | 23.6686 mL | 47.3373 mL | 59.1716 mL |
| 5 mM | 0.4734 mL | 2.3669 mL | 4.7337 mL | 9.4675 mL | 11.8343 mL |
| 10 mM | 0.2367 mL | 1.1834 mL | 2.3669 mL | 4.7337 mL | 5.9172 mL |
| 50 mM | 0.0473 mL | 0.2367 mL | 0.4734 mL | 0.9467 mL | 1.1834 mL |
| 100 mM | 0.0237 mL | 0.1183 mL | 0.2367 mL | 0.4734 mL | 0.5917 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Eleutheroside B1
Catalog No.:BCN0267
CAS No.:16845-16-2
- (-)-Conduritol F
Catalog No.:BCN0266
CAS No.:6090-98-8
- Cynandione A
Catalog No.:BCN0265
CAS No.:168706-29-4
- Wilfoside C 1N
Catalog No.:BCN0264
CAS No.:96829-53-7
- Bungeiside B
Catalog No.:BCN0263
CAS No.:149561-88-6
- Wilfoside K 1N
Catalog No.:BCN0262
CAS No.:100802-91-3
- (Z)-5-(Heptadec-10-en-1-yl)resorcinol
Catalog No.:BCN0261
CAS No.:52483-21-3
- Belamcandol B
Catalog No.:BCN0260
CAS No.:137786-94-8
- Deoxy euphorbia factor L1
Catalog No.:BCN0259
CAS No.:247099-01-0
- Euphorbia factor L22
Catalog No.:BCN0258
CAS No.:1613700-09-6
- Planispine A
Catalog No.:BCN0257
CAS No.:1202761-42-9
- Secotubeimoside I
Catalog No.:BCN0256
CAS No.:106235-32-9
- Dihydrocapsiate
Catalog No.:BCN0269
CAS No.:205687-03-2
- Nordihydrocapsiate
Catalog No.:BCN0270
CAS No.:220012-53-3
- Colnelenic acid
Catalog No.:BCN0271
CAS No.:52591-16-9
- Capsiate
Catalog No.:BCN0272
CAS No.:205687-01-0
- Vaticanol B
Catalog No.:BCN0273
CAS No.:287101-83-1
- Soyasaponin III
Catalog No.:BCN0287
CAS No.:55304-02-4
- Soyasaponin A3
Catalog No.:BCN0288
CAS No.:114077-04-2
- Soyasaponin A1
Catalog No.:BCN0289
CAS No.:78693-94-4
- Vatalbinoside A
Catalog No.:BCN0274
CAS No.:
- Vatalbinoside C
Catalog No.:BCN0275
CAS No.:
- Vatalbinoside G
Catalog No.:BCN0276
CAS No.:
- Vatalbinoside H
Catalog No.:BCN0277
CAS No.:
Iridoids from Valeriana jatamansi with anti-inflammatory and antiproliferative properties.[Pubmed:33548771]
Phytochemistry. 2021 Apr;184:112681.
Seven undescribed (valejatadoids A-G) and 26 known iridoids were obtained from the roots and rhizomes of Valeriana jatamansi. Their structures were determined based on extensive spectroscopic data, especially 1D and 2D NMR, along with HRESIMS. Valejatadoid B is a monoene-type iridoid with a unique double bond between C-4 and C-5. Valejatadoids D-G, jatamanin U, jatamanin O, jatamanvaltrate E, valeriotetrate C, IVHD-valtrate, 10-isovaleroxy-valtrathydrin, jatamanvaltrate Q, valeriandoid F, jatamanvaltrate K, jatamanvaltrate W and Isovaltrate were more potent than the positive control when evaluated for inhibition of NO production. Among them, valeriandoid F and jatamanvaltrate K exhibited the most significant inhibitory effects with IC50 values of 0.88 and 0.62 muM, respectively. In addition, valeriandoid F selectively inhibited the proliferation of human glioma stem cell lines, GSC-3# and GSC-18#, with IC50 values of 7.16 and 5.75 muM, respectively.
Induction of cytotoxicity and apoptosis in human gastric cancer cell SGC-7901 by isovaltrate acetoxyhydrin isolated from Patrinia heterophylla bunge involves a mitochondrial pathway and G2/M phase cell cycle arrest.[Pubmed:24377554]
Asian Pac J Cancer Prev. 2013;14(11):6481-6.
BACKGROUND: Our previous study demonstrated cytotoxicity of a crude extract from Patrinia heterophylla Bunge (PHEB). In the present study, we aimed to investigate the effects of Isovaltrate acetoxyhydrin (IA) isolated from PHEB on the gastric cancer cell SGC-7901, in order to explore a potential treatment for gastric cancer. METHODS: MTT assays were employed to determine the effects of IA on cell vitality and proliferation, with monitoring of cell morphology changes and examination of apoptosis with Annexin V-PI staining. Flow cytometry was used to assess cell cycle progression and mitochondrial membrane potential. The activity of caspase 3, 9 was evaluated by spectrophotometry, and the protein levels of Bax, Bcl2 and Cyclin B1 were analyzed with Western blotting of total proteins extracted from cultured cells. RESULTS: The results demonstrated direct toxicity of IA towards SGC-7901 cells. Evidence of apoptosis included blebbing and chromatin condensation. Annexin V-PI assays revealed early apoptosis, involving rapid depolarization of mitochondrial membranes and activity of caspase 3, 9 signaling pathways. Western blotting showed that Bcl2 and Bax proteins was down- and up-regulated, respectively, and cyclin B1 was up-regulated. Cell cycle analysis further indicated that IA could induce G2/M phase arrest in SGC-7901 cells. CONCLUSIONS: In conclusion, we believe that IA induces apoptosis of SGC-7901 cells, therefore providing a potential therapeutic agent for treatment of gastric cancer.
Effect of storage time and conditions on the diene valepotriates content of the extract of Valeriana glechomifolia obtained by supercritical carbon dioxide.[Pubmed:21953720]
Phytochem Anal. 2012 May-Jun;23(3):222-7.
INTRODUCTION: Valepotriates (epoxy iridoid esters) represent an important group of constituents that contribute to pharmacological effects for the genus Valeriana. Storage and extraction of valepotriates is a demanding task, as these compounds are thermolabile and unstable: even when decomposition products are not formed, isovaleric acid liberation from the iridoid nucleus originate compounds with less complex substituents. OBJECTIVE: To study the influence of time and storage conditions on the diene valepotriates (valtrate, Isovaltrate, acevaltrate, 1-beta-acevaltrate, 1-beta-aceacevaltrate) content of the Valeriana glechomifolia (native to southern Brazil), extract was obtained by supercritical fluid extraction using CO(2) as the fluid (SF-CO(2)). METHODOLOGY: Above-ground and below-ground material of V. glechomifolia was extracted by SF-CO(2) (40 degrees C, 90 bar). The extract was stored under nitrogen atmosphere or solubilised in methanol. Valepotriates stability was accessed during storage at -20 degrees C over 8 months through reverse-phase HPLC (mobile phase acetonitrile:water 50:50 (v/v); 254 nm). RESULTS: A gradual increase in valtrate levels and decrease in acevaltrate, 1-beta-acevaltrate and 1-beta-aceacevaltrate, concentration were observed from the first month of storage for the dry extract. However, for the methanol solubilised extract these changes occurred only after the third month and were accompanied by reduction in Isovaltrate levels and formation of decomposition products. CONCLUSION: SF-CO(2) showed high selectivity for valepotriates extraction. This is the first report on valepotriates molecular conversion, which was less accelerated when the extract was stored in methanol, but under this condition degradation products are also present, probably baldrinals, that are not observed in the dry extract.
Sorbifolivaltrates A-D, diene valepotriates from Valeriana sorbifolia(1).[Pubmed:18052324]
J Nat Prod. 2007 Dec;70(12):2045-8.
Four new diene valepotriates, sorbifolivaltrates A-D ( 1- 4), and the known compounds Isovaltrate ( 5), valtrate ( 6), seneciovaltrate ( 7), valtrate hydrine B3 ( 8), and valtrate hydrine B7 ( 9), have been isolated by bioassay-guided fractionation of the cytotoxic hexanes and methyl ethyl ketone crude extracts of the aerial parts of Valeriana sorbifolia occurring in the Sonoran desert. The structures of 1- 4 were determined on the basis of their high-resolution mass spectrometric and NMR spectroscopic data. All compounds exhibited weak to moderate cytotoxicity against the human metastatic prostate cancer cell line, PC-3M.
Interaction of valerian extracts of different polarity with adenosine receptors: identification of isovaltrate as an inverse agonist at A1 receptors.[Pubmed:17097622]
Biochem Pharmacol. 2007 Jan 15;73(2):248-58.
A series of extracts of valerian roots (Valeriana officinalis L.) was prepared with solvents of different polarity. Polar as well as nonpolar extracts were found to interact with adenosine A(1) receptors. While polar extracts activated A(1) receptors (partial agonistic activity), nonpolar extracts showed antagonistic or inverse agonistic activity at A(1) receptors, as demonstrated by GTPgammaS binding assays at human recombinant A(1) receptors stably expressed in Chinese hamster ovary (CHO) cells. Guided by radioligand binding assays, fractionation of a lipophilic petroleum ether:diethyl ether (1:1) extract led to the isolation of Isovaltrate, which was characterized as a potent, highly efficacious inverse agonist at adenosine A(1) receptors (K(i) rat A(1): 2.05 microM). In experiments at rat brain slices measuring post-synaptic potentials (PSPs) in cortical neurons, Isovaltrate at least partly reversed the reduction in the PSPs induced by the adenosine A(1) receptor agonist N(6)-cyclopentyladenosine (CPA). Isovaltrate may serve as a new lead structure for the development of inverse agonists at adenosine A(1) receptors. The common use of hydrophilic, but not lipophilic valerian extracts as mild sleep-inducing agents is consistent with the opposite actions of hydrophilic and lipophilic extracts on adenosine receptors.
Preserved pharmacological activity of hepatocytes-treated extracts of valerian and St. John's wort.[Pubmed:16041642]
Planta Med. 2005 Jul;71(7):592-8.
The two herbal extracts valerian (Valeriana officinalis L.) and St. John's wort (Hypericum perforatum L.) were studied for their metabolic changes upon incubation with freshly prepared rat hepatocytes and subsequently analysed phytochemically as well as pharmacologically in vitro. Quantitative HPLC analysis of valerian extracts revealed considerable metabolic activities with regard to sesquiterpenes and iridoids. The amount of acetoxyvalerenic acid decreased 9-fold, while that of hydroxyvalerenic acid correspondingly increased 9-fold due to O-deacetylation. The valepotriates didrovaltrate, Isovaltrate and valtrate decreased 2-, 18- and 16-fold, respectively. However, the binding affinities of the incubated extracts to the benzodiazepine and picrotoxin binding site of the GABA (A) receptor were quite similar to those of the non-incubated extracts. Neither valerenic acids nor valepotriates exhibited any significant effect on the two binding sites when tested as single compounds. Therefore, either other constituents represent the active ones or multiple compounds are necessary for the observed inhibitory and allosteric effects at the GABA (A) receptor. Extracts of St. John's wort were less potently metabolised than valerian. The amount of pseudohypericin and the main flavonoids (hyperoside, rutin, isoquercitrin, quercitrin, quercetin and I3,II8-biapigenin) slightly decreased during the 4-h incubation period. Both the antagonist effect at the corticotropin-releasing factor (CRF) type 1 receptor and the binding inhibition at the 5-HT transporter were attenuated during the metabolic treatment. The reduced antagonist effect correlates with the decreasing amount of pseudohypericin known to be a CRF (1) receptor antagonist. In conclusion, the incubation of plant extracts with freshly prepared rat hepatocytes represents a useful approach to study the pharmacological action of metabolised plant extracts. The consistent pharmacological activity of both valerian and St. John's wort is concordant with the known clinical efficacy of pharmacological activities.
Cytotoxic potential of valerian constituents and valerian tinctures.[Pubmed:23195845]
Phytomedicine. 1998 May;5(3):219-25.
Underground parts of three Valeriana species, namely V. officinalis L. s.l., V. wallichii DC. (V. jatamansi Jones), and V. edulis Nutt. ex Torr & Gray ssp. procera (H.B.K.) F. G. Meyer (V. mexicana DC.), are used in phytotherapy because of their mild sedative properties. Characteristic constituents of these species, which are regarded also as the active principles, were tested for cytotoxicity against GLC(4), a human small-cell lung cancer cell line, and against COLO 320, a human colorectal cancer cell line, using the microculture tetrazolium (MTT) assay. Valepotriates of the diene type (valtrate, Isovaltrate and acevaltrate) displayed the highest cytotoxicity, with IC50 values of 1-6 muM, following continuous incubation. The monoene type valepotriates (didrovaltrate and isovaleroxyhydroxydidrovaltrate) were 2- to 3-fold less toxic. Baldrinal and homobaldrinal, decomposition products of valepotriates, were 10- to 30-fold less toxic than their parent compounds. Isovaltral had a higher cytotoxicity than its parent compound Isovaltrate. Valerenic acids (valerenic acid, acetoxyvalerenic acid, hydroxyvalerenic acid and methyl valerenate), which are characteristic for V. officinalis, had a low toxicity with IC(50) values between 100 and 200 muM. Freshly prepared and stored tinctures, prepared from roots and rhizomes of the three valerian species, were analysed for valepotriates, baldrinals and valerenic acids, and also tested for cytotoxicity. There was a clear relationship between the valepotriate contents of the freshly prepared tinctures and their toxicity. Upon storage, valepotriates decomposed, which was reflected in a significant reduction of the cytotoxic effect.
Production of valepotriates by hairy root cultures of Centranthus ruber DC.[Pubmed:24186763]
Plant Cell Rep. 1995 Feb;14(5):294-8.
Hairy root cultures of Centranthus ruber DC. were established by infection of sterile plantlets with Agrobacterium rhizogenes, strain R1601. The transformed roots were grown in 12 different, hormone-free liquid media, and valtrate, Isovaltrate, 7-desisovaleroyl-7-acetylvaltrate, 7-homovaltrate, didrovaltrate and isovaleroxyhydroxydidrovaltrate were quantified by high performance liquid chromatography. The highest overall valepotriate content (3.0% dry wt) was observed in half-strength Gamborg B5 medium supplemented with 3% sucrose. This concentration is very similar to that found in the roots of parent plants grown in the field. The use of N,N-dimethylmorpholinium iodide, a plant bioregulator, was very detrimental to the hairy root growth and to the valepotriate production. The hairy roots cultured in half strength Gamborg B5 liquid medium supplemented with 3% sucrose for 45 days produced over 31 mg/g dry wt valepotriates.
High-yield production of valepotriates by hairy root cultures of Valeriana officnalis L. var. sambucifolia Mikan.[Pubmed:24201434]
Plant Cell Rep. 1992 Jul;11(7):339-42.
Hairy root cultures of Valeriana officinalis var. sambucifolia were established by infection of sterile plantlets with Agrobacterium rhizogenes strain R1601 The transformed roots were grown in 10 different, hormone-free liquid media and the Isovaltrate, valtrate, didrovaltrate, isovaleroxyhydroxydidrovaltrate content was quantified by HPLC. Valepotriates were entirely retained inside the root tissues. The highest overall valepotriate content (10.3 % dry wt), 4 times the amount found in the roots of 9-month-old nontransformed plants, was observed in half strength Gamborg B5 medium supplemented with 2 % sucrose. The hairy roots cultured in Murashige and Skoog liquid medium supplemented with 2 % sucrose for 50 days produced over 44 mg/g dry wt valepotriates.
Bacterial mutagenicity of the tranquilizing constituents of Valerianaceae roots.[Pubmed:3511364]
Mutat Res. 1986 Jan-Feb;169(1-2):23-7.
The valepotriates valtrate/Isovaltrate and dihydrovaltrate are considered to be the main tranquilizing constituents of drugs derived from the roots of several Valerianaceae. The decomposition products of valtrate and Isovaltrate include the metabolites baldrinal and homobaldrinal, respectively, whereas the decomposition products of dihydrovaltrate do not include baldrinal-like metabolites. Purified valtrate/Isovaltrate, dihydrovaltrate, baldrinal and homobaldrinal were investigated for their genotoxic activity in the Salmonella/microsome test and the SOS-chromotest. The valepotriates developed mutagenic activity in these test systems only in the presence of S9 mix, whereas both baldrinals showed mutagenic effects in both tests with and without metabolic activation.
In vitro mutagenicity of valepotriates.[Pubmed:3994511]
Arch Toxicol. 1985 Feb;56(4):267-71.
Valepotriates are epoxide-bearing triesters of the monoterpene alcohol 4,7-dimethylcyclopenta-(c)-pyrane isolated from the roots of several Valerianacae species. They are regarded as the main tranquilizing constituents of these drugs. Although the valepotriates valtrate/Isovaltrate (VAL) and dihydrovaltrate (DH-VAL) showed a strong alkylating activity against the nucleophilic agent 4-(p-nitrobenzyl)-pyridine (NBP), they were not clearly mutagenic for the strains TA98, TA100, TA1535, and TA1537 of Salmonella typhimurium or for the strains WP2 and WP2 uvrA- of Escherichia coli in the absence of a metabolic activation system (S9-mix). However, the valepotriates were mutagenic for TA100, WP2 and WP2 uvrA- at concentrations up to about 1.0 mumole/plate when S9-mix was added to the test system. With more than 1 mumole/plate the valepotriates were toxic in the presence of a metabolic activation system for all strains tested. The mutagenicity of the valepotriates was inversely related to the protein content of the S9-mix used. The mutagenicity and toxicity of the valepotriates could be inhibited when the S9-mix was preincubated with the esterase inhibitor paraoxon (1 mM) for 5 min before the test compounds and bacteria were added. Therefore, bioactivation of the valepotriates by an enzymatic hydrolysis of their ester groups is considered. This could be proven by activating the valepotriates with purified esterase.
The Structure of New Valepotriates from Tissue Cultures of Valeriana wallichii.[Pubmed:17340305]
Planta Med. 1984 Jun;50(3):245-8.
Colchicin-treated tissue cultures of VALERIANA WALLICHII produced besides the known valepotriates homovaltrate, Isovaltrate, valtrate, acevaltrate and didrovaltrate several unknown substances of diene-valepotriate structure. Nine genuine valepotriates and two degradation products were isolated and their structure elucidated by means of their (13)C-NMR spectra.
Influence of valtrate/isovaltrate on the hematopoiesis and metabolic liver activity in mice in vivo.[Pubmed:17340233]
Planta Med. 1984 Feb;50(1):1-4.
Recently, cytotoxic effects of valepotriates with an epoxide moiety have been described on mouse bone marrow early progenitor cells IN VITRO. Consequently, the possible IN VIVO toxicity of valtrate on hematopoietic precursor cells was investigated. Mice were treated i.p. with 45 and 65 mg/kg or p.o. with 45 and 1350 mg/kg of the drug. Three days after treatment, colony formation of progenitor cells (CFC-S, GM-CFC, E-CFC) was not significantly different for control and experimental groups. Furthermore, the effect of valtrate on the ability of the liver to metabolize [ (14)C]methacetin was investigated by measuring the (14)CO (2) exhalation (breath test). There was a distinct reduction of the initial exhalation of (14)CO (2) following i.p. injection of 50 mg/kg of valtrate, but no effect was found after 50 or 1500 mg/kg of p.o. These results suggest that toxicity of valtrate in vivo is restricted because the distribution of the drug via circulation is obviously small.
Antispasmodic effects of valeriana compounds: an in-vivo and in-vitro study on the guinea-pig ileum.[Pubmed:7114974]
Arch Int Pharmacodyn Ther. 1982 Jun;257(2):274-87.
The valepotriates Isovaltrate and valtrate, and the essential oil compound valeranone caused a suppression of rhythmic contractions in a closed part of the guinea-pig ileum in-vivo. The same compounds and didrovaltrate relaxed potassium stimulated contractures and inhibited BaCl2 contractions in guinea-pig ileum preparations in-vitro. Guinea-pig stomach fundic strips stimulated by carbachol were also relaxed by these substances. Potassium stimulated smooth muscle cells were also relaxed by the valeriana compounds (10(-5)-10(-4) M) even, when autonomic receptors were blocked by appropriate antagonists. In lower concentrations (10(-6)-10(-5) M), the compounds did not affect the dose-response curves of carbachol and isoprenaline. In some experiments valeranone at 4.10(-6) M produced an increased isoprenaline relaxation of the guinea-pig ileum. Valeranone and didrovaltrate were about equipotent to papaverine in inhibiting BaCl2 contractions. It is concluded that the valeriana compounds probably relax stimulated smooth muscle cells by acting as musculotropic agents and not by interacting with receptors of the autonomic nervous system.


