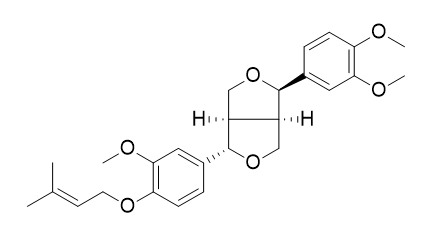
Planispine ACAS# 1202761-42-9 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 1202761-42-9 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C26H32O6 | M.Wt | 440.5 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Planispine A Dilution Calculator

Planispine A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2701 mL | 11.3507 mL | 22.7015 mL | 45.403 mL | 56.7537 mL |
| 5 mM | 0.454 mL | 2.2701 mL | 4.5403 mL | 9.0806 mL | 11.3507 mL |
| 10 mM | 0.227 mL | 1.1351 mL | 2.2701 mL | 4.5403 mL | 5.6754 mL |
| 50 mM | 0.0454 mL | 0.227 mL | 0.454 mL | 0.9081 mL | 1.1351 mL |
| 100 mM | 0.0227 mL | 0.1135 mL | 0.227 mL | 0.454 mL | 0.5675 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Secotubeimoside I
Catalog No.:BCN0256
CAS No.:106235-32-9
- Fuzitine
Catalog No.:BCN0255
CAS No.:142287-96-5
- Euphorbia factor L25
Catalog No.:BCN0254
CAS No.:303174-98-3
- Peiioside B
Catalog No.:BCN0253
CAS No.:1610618-91-1
- Gynosaponin TN2
Catalog No.:BCN0252
CAS No.:77658-95-8
- 4'-O-Methyllucenin II (Diosmetin 6,8-di-C-glucoside)
Catalog No.:BCN0251
CAS No.:98813-28-6
- Dioscoreside E
Catalog No.:BCN0250
CAS No.:435321-73-6
- Dioscoreside C
Catalog No.:BCN0249
CAS No.:344912-80-7
- O-Acetylgalanthamine
Catalog No.:BCN0248
CAS No.:25650-83-3
- Galanthamine 10-Oxide
Catalog No.:BCN0247
CAS No.:134332-50-6
- Anhydrobyankangelicin
Catalog No.:BCN0246
CAS No.:35214-81-4
- O-Desmethyl galanthamine
Catalog No.:BCN0245
CAS No.:60755-80-8
- Euphorbia factor L22
Catalog No.:BCN0258
CAS No.:1613700-09-6
- Deoxy euphorbia factor L1
Catalog No.:BCN0259
CAS No.:247099-01-0
- Belamcandol B
Catalog No.:BCN0260
CAS No.:137786-94-8
- (Z)-5-(Heptadec-10-en-1-yl)resorcinol
Catalog No.:BCN0261
CAS No.:52483-21-3
- Wilfoside K 1N
Catalog No.:BCN0262
CAS No.:100802-91-3
- Bungeiside B
Catalog No.:BCN0263
CAS No.:149561-88-6
- Wilfoside C 1N
Catalog No.:BCN0264
CAS No.:96829-53-7
- Cynandione A
Catalog No.:BCN0265
CAS No.:168706-29-4
- (-)-Conduritol F
Catalog No.:BCN0266
CAS No.:6090-98-8
- Eleutheroside B1
Catalog No.:BCN0267
CAS No.:16845-16-2
- Isovaltrate
Catalog No.:BCN0268
CAS No.:31078-10-1
- Dihydrocapsiate
Catalog No.:BCN0269
CAS No.:205687-03-2
Two new lignans from Zanthoxylum armatum.[Pubmed:33289429]
Nat Prod Res. 2020 Dec 8:1-6.
Zanthoxylum armatum, its peels possessed better special flavour, as well as various bioactivities, such as anti-inflammatory, anti-microbial and anti-tumour. In our chemical investigation on the peels of Z. armatum, two new lignans (1 and 2) and three known lignans (3-5) were isolated by silica gel column chromatography, ODS column and preparative HPLC and their structures were established as zanthlignans A and B (1-2), (-)-asarinin (3), phylligenin (4) and Planispine A (5) through various spectroscopic techniques including UV, IR, HR-ESI-MS, NMR and CD methods.
Antinociceptive and anti-inflammatory activities of ethyl acetate fraction from Zanthoxylum armatum in mice.[Pubmed:21059381]
Fitoterapia. 2011 Apr;82(3):347-51.
Zanthoxylum armatum DC. is a traditional Chinese medicine that is prescribed to alleviate pain and treat inflammatory disorders. This species is distributed mainly in the southeast and southwest regions of China. In the present study, we found that ethyl acetate fraction of ethanolic extract of Z. armatum could significantly decrease acetic acid-induced writhing numbers, and suppress formalin induced licking times in the first phase at the highest dose and in the second phase at all tested doses. This observation revealed that Z. armatum extract possessed powerful antinociceptive activity. The mechanisms of the antinociceptive effect might be mainly involved in the periphery inflammatory analgesic. In addition, the ethyl acetate fraction also inhibited xylene-induced ear swelling in a dose-dependent manner in mice. Eight lignans [eudesmin, horsfieldin, fargesin, kobusin, sesamin, asarinin, Planispine A, and pinoresinol-di-3,3-dimethylallyl] were identified as major components of the ethyl acetate fraction. Considering related studies reporting the anti-inflammatory activity for the identified lignans, lignan might be responsible for its anti-inflammatory activity. Our results confirm that the traditional use of Z. armatum in the treatment of inflammation and pain is warranted.


