Belamcandol BCAS# 137786-94-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 137786-94-8 | SDF | Download SDF |
| PubChem ID | 10969651 | Appearance | Powder |
| Formula | C22H36O2 | M.Wt | 332.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
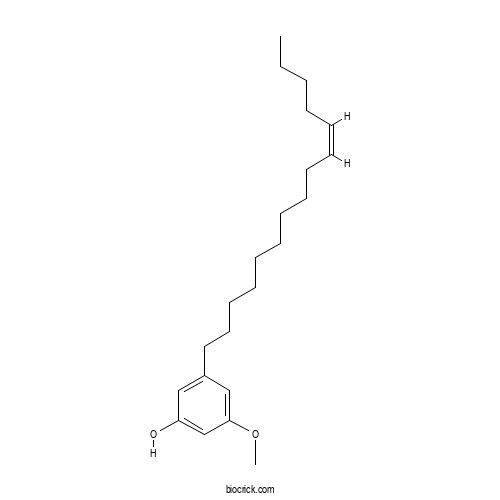
| Chemical Name | 3-methoxy-5-[(Z)-pentadec-10-enyl]phenol | ||
| SMILES | CCCCC=CCCCCCCCCCC1=CC(=CC(=C1)OC)O | ||
| Standard InChIKey | NKOPRUNFJQCUCF-SREVYHEPSA-N | ||
| Standard InChI | InChI=1S/C22H36O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-20-17-21(23)19-22(18-20)24-2/h6-7,17-19,23H,3-5,8-16H2,1-2H3/b7-6- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Belamcandol B Dilution Calculator

Belamcandol B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0075 mL | 15.0376 mL | 30.0752 mL | 60.1504 mL | 75.188 mL |
| 5 mM | 0.6015 mL | 3.0075 mL | 6.015 mL | 12.0301 mL | 15.0376 mL |
| 10 mM | 0.3008 mL | 1.5038 mL | 3.0075 mL | 6.015 mL | 7.5188 mL |
| 50 mM | 0.0602 mL | 0.3008 mL | 0.6015 mL | 1.203 mL | 1.5038 mL |
| 100 mM | 0.0301 mL | 0.1504 mL | 0.3008 mL | 0.6015 mL | 0.7519 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Deoxy euphorbia factor L1
Catalog No.:BCN0259
CAS No.:247099-01-0
- Euphorbia factor L22
Catalog No.:BCN0258
CAS No.:1613700-09-6
- Planispine A
Catalog No.:BCN0257
CAS No.:1202761-42-9
- Secotubeimoside I
Catalog No.:BCN0256
CAS No.:106235-32-9
- Fuzitine
Catalog No.:BCN0255
CAS No.:142287-96-5
- Euphorbia factor L25
Catalog No.:BCN0254
CAS No.:303174-98-3
- Peiioside B
Catalog No.:BCN0253
CAS No.:1610618-91-1
- Gynosaponin TN2
Catalog No.:BCN0252
CAS No.:77658-95-8
- 4'-O-Methyllucenin II (Diosmetin 6,8-di-C-glucoside)
Catalog No.:BCN0251
CAS No.:98813-28-6
- Dioscoreside E
Catalog No.:BCN0250
CAS No.:435321-73-6
- Dioscoreside C
Catalog No.:BCN0249
CAS No.:344912-80-7
- O-Acetylgalanthamine
Catalog No.:BCN0248
CAS No.:25650-83-3
- (Z)-5-(Heptadec-10-en-1-yl)resorcinol
Catalog No.:BCN0261
CAS No.:52483-21-3
- Wilfoside K 1N
Catalog No.:BCN0262
CAS No.:100802-91-3
- Bungeiside B
Catalog No.:BCN0263
CAS No.:149561-88-6
- Wilfoside C 1N
Catalog No.:BCN0264
CAS No.:96829-53-7
- Cynandione A
Catalog No.:BCN0265
CAS No.:168706-29-4
- (-)-Conduritol F
Catalog No.:BCN0266
CAS No.:6090-98-8
- Eleutheroside B1
Catalog No.:BCN0267
CAS No.:16845-16-2
- Isovaltrate
Catalog No.:BCN0268
CAS No.:31078-10-1
- Dihydrocapsiate
Catalog No.:BCN0269
CAS No.:205687-03-2
- Nordihydrocapsiate
Catalog No.:BCN0270
CAS No.:220012-53-3
- Colnelenic acid
Catalog No.:BCN0271
CAS No.:52591-16-9
- Capsiate
Catalog No.:BCN0272
CAS No.:205687-01-0
Estrogenic phytochemical from Labisia pumila (Myrsinaceae) with selectivity towards estrogen receptor alpha and beta subtypes.[Pubmed:31295513]
Fitoterapia. 2019 Sep;137:104256.
Labisia pumila var. alata (Myrsinaceae) or "Kacip fatimah" is a famous Malay traditional herb used for the maintenance of women's health. The extracts of L.pumila displayed estrogenic activity in rats. Nonetheless, the estrogenic bioactives were not identified. The aim of the study is to identify estrogenic compounds contributing to the established estrogenic activity. Bioactivity-guided-isolation method guided the isolation of pure bioactives. The hexane extract was subjected to a series of silica gel flash and open column chromatography with increasing amount of ethyl acetate in hexane or methanol in chloroform. Each fraction or pure compounds were evaluated on it's estrogen receptor (ER) binding activity with the fluorescence polarization competitive ERalpha and ERbeta binding assay kit. Cytotoxic assay using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay method was used to establish the cytotoxic activity of the compounds. Four alkyl resorcinols and a dimeric 1,4-benzoquinone, namely Belamcandol B (1), 5-pentadec-10'-(Z)-enyl resorcinol (2), 1,3-dihydroxy-5-pentadecylbenzene (3), 5-(heptadec-12'-(Z)-enyl) resorcinol (4) and demethylbelamcandaquinone B (5) were identified with selective binding affinities towards either ERalpha or ERbeta exhibiting selectivity ratio from 0.15-11.9. Alkyl resorcinols (2)-(4) exhibited cytotoxic activity towards HL60 cells with IC50 values from 19.5-22.0muM. Structural differences between compounds influence the binding affinities to ER subtypes. Further study is needed to establish the agonist or antagonist effect of these compounds on various tissues and to identify if these compounds exert cytotoxic activity through the ERs. When consuming L.pumila as a complementary medicine, careful consideration regarding it's estrogenic compound content should be given due consideration.
Quantitative determination of triperpene saponins and alkenated-phenolics from Labisia pumila using an LC-UV/ELSD method and confirmation by LC-ESI-TOF.[Pubmed:21590653]
Planta Med. 2011 Oct;77(15):1742-8.
This study describes the first analytical method for the determination of four triterpene saponins (ardisicrenoside B, ardisiacrispin A, 3- O- alpha- L-rhamnopyranosyl-(1 --> 2)-beta-D-glucopyranosyl-(1 --> 4)-alpha-L-arabinopynanosyl cyclamiretin A and ardisimamilloside H) and three alkenated-phenolics (irisresorcinol, Belamcandol B, and demethylbelamcandaquinone B) from the leaves, leaves/stems, and roots of LABISIA PUMILA using an HPLC-UV-ELSD method. The separation was achieved using a reversed-phase (C-18) column, PDA and ELS detection, and a water/acetonitrile gradient as the mobile phase. The major triterpenoid (ardisiacrispin A) and irisresorcinol compounds were detected at a concentration as low as 10.0 and 0.2 microg/mL, respectively. Analysis of various samples showed considerable variation of 0.11-2.46 % for the major triterpenoid compound, ardisiacrispin A. LC-mass spectrometry method coupled with electrospray ionization (ESI) is described for the identification of compounds in plant samples. This method involved the use of the [M + Na]+ and [M + NH(4)]+ ions for compounds 1-4 in the positive ion mode with extractive ion chromatogram (EIC).
Naturally occurring 5-lipoxygenase inhibitor. II. Structures and syntheses of ardisianones A and B, and maesanin, alkenyl-1,4-benzoquinones from the rhizome of Ardisia japonica.[Pubmed:8477510]
Chem Pharm Bull (Tokyo). 1993 Mar;41(3):561-5.
New alkenyl-1,4-benzoquinones, ardisianones A (1) and B (2), and the known maesanin (3) as 5-lipoxygenase inhibitors have been isolated from the rhizome of Ardisia japonica. Their structures have been elucidated as 2-methoxy-6-[(Z)-10'-pentadecenyl]-1,4-benzoquinone and 5-hydroxy-2-methoxy-6-[(Z)-8'-tridecenyl]-1,4-benzoquinone, respectively, on the basis of spectroscopic data and chemical degradation. Ardisianone A (1), maesanin (3) and belamcandol A (7) have been synthesized starting from Belamcandol B (6), readily prepared by Wittig reaction between 9-(2-tetrahydropyranyloxy)nonanal and 3,5-dimethoxybenzyltriphenylphsophonium bromide followed by selective demethylation with sodium thioethoxide.


