BakuchicinCAS# 4412-93-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4412-93-5 | SDF | Download SDF |
| PubChem ID | 3083848 | Appearance | White powder |
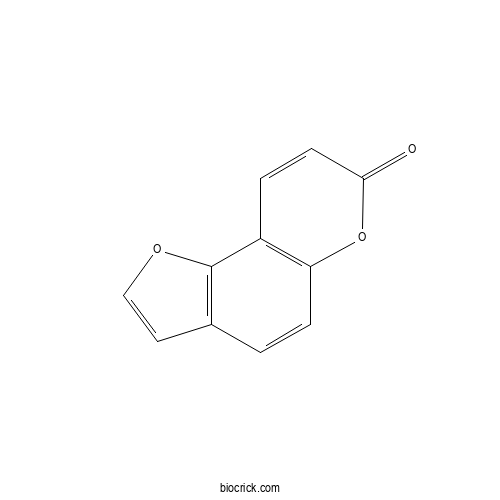
| Formula | C11H6O3 | M.Wt | 186.16 |
| Type of Compound | Phenylpropanes | Storage | Desiccate at -20°C |
| Synonyms | Allopsoralen | ||
| Solubility | Soluble in chloroform and methanol; insoluble in water | ||
| Chemical Name | furo[2,3-f]chromen-7-one | ||
| SMILES | C1=CC2=C(C=CC(=O)O2)C3=C1C=CO3 | ||
| Standard InChIKey | HMUJHZNYHJMOHR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H6O3/c12-10-4-2-8-9(14-10)3-1-7-5-6-13-11(7)8/h1-6H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Bakuchicin Dilution Calculator

Bakuchicin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.3717 mL | 26.8586 mL | 53.7172 mL | 107.4345 mL | 134.2931 mL |
| 5 mM | 1.0743 mL | 5.3717 mL | 10.7434 mL | 21.4869 mL | 26.8586 mL |
| 10 mM | 0.5372 mL | 2.6859 mL | 5.3717 mL | 10.7434 mL | 13.4293 mL |
| 50 mM | 0.1074 mL | 0.5372 mL | 1.0743 mL | 2.1487 mL | 2.6859 mL |
| 100 mM | 0.0537 mL | 0.2686 mL | 0.5372 mL | 1.0743 mL | 1.3429 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Avenanthramide D
Catalog No.:BCN0301
CAS No.:115610-36-1
- Atropine N-oxide hydrochloride
Catalog No.:BCN0300
CAS No.:4574-60-1
- Apoatropine hydrochloride
Catalog No.:BCN0299
CAS No.:5978-81-4
- (-)-Antofine
Catalog No.:BCN0298
CAS No.:32671-82-2
- Anisodine hydrobromide
Catalog No.:BCN0297
CAS No.:76822-34-9
- Anemonin
Catalog No.:BCN0296
CAS No.:508-44-1
- Andrograpanin
Catalog No.:BCN0295
CAS No.:82209-74-3
- L-(±)-Alliin
Catalog No.:BCN0294
CAS No.:17795-26-5
- Procyanidin A4
Catalog No.:BCN0293
CAS No.:111466-29-6
- Ergocornine
Catalog No.:BCN0292
CAS No.:564-36-3
- Agroclavine
Catalog No.:BCN0291
CAS No.:548-42-5
- Roridin A
Catalog No.:BCN0290
CAS No.:14729-29-4
- Ballonigrine
Catalog No.:BCN0303
CAS No.:62340-62-9
- Berberine chloride dihydrate
Catalog No.:BCN0304
CAS No.:5956-60-5
- Californidine perchlorate
Catalog No.:BCN0305
CAS No.:17939-31-0
- Cannflavin A
Catalog No.:BCN0306
CAS No.:76735-57-4
- Castalagin
Catalog No.:BCN0307
CAS No.:24312-00-3
- (-)-Catechin
Catalog No.:BCN0308
CAS No.:18829-70-4
- Convolidine
Catalog No.:BCN0309
CAS No.:63911-32-0
- trans-p-Coumaric acid
Catalog No.:BCN0310
CAS No.:501-98-4
- trans-Coutaric acid
Catalog No.:BCN0311
CAS No.:27174-07-8
- Cycloartenol
Catalog No.:BCN0312
CAS No.:469-38-5
- 5,6-Dehydro 7,8-dihydrokavain
Catalog No.:BCN0313
CAS No.:3155-51-9
- 23-epi-26-Deoxyactein
Catalog No.:BCN0314
CAS No.:501938-01-8
Bakuchicin attenuates atopic skin inflammation.[Pubmed:32768955]
Biomed Pharmacother. 2020 Sep;129:110466.
Psoralea corylifolia is a medicinal herb that provides advantageous pharmacological effects against vitiligo and skin rash. Former studies have shown that Bakuchicin, a furanocoumarin compound from the fruits of P. corylifolia, has therapeutic effects against inflammation, and infection. This study aimed to define the pharmacological effects of Bakuchicin on inflammatory responses and lichenification, the major symptoms of atopic dermatitis (AD). To induce AD-like skin inflammation, we exposed the ears of female BALB/c mice to 2, 4-dinitrochlorobenzene (DNCB) and Dermatophagoides farinae (house dust mite) extract (DFE) for 4 weeks. Intragastric administration of Bakuchicin attenuated the symptoms of AD-like skin inflammation, as evident by reductions in ear thickness, erythema, and keratosis. Bakuchicin also reversed increases in auricular epidermal and dermal layer thicknesses, and attenuated eosinophil and mast cell infiltration in AD-induced mice. It also suppressed Th2 gene expression as well as that of pro-inflammatory cytokines and chemokines, such as interleukin (IL)-4, IL-13, IL-31, IL-1beta, IL-6, CXCL-1, and CCL-17 in the ear tissue. The levels of total and DFE-specific immunoglobulin (Ig)E, and IgG2a in the mice sera were reduced by the Bakuchicin. To investigate the effect of Bakuchicin on keratinocytes, experiments were performed using HaCaT cells, the representative cell type used in skin disease studies. Tumor necrosis factor-alpha and interferon-gamma were used to activate keratinocytes. Bakuchicin suppressed Th2 gene expression and that of pro-inflammatory cytokines and chemokines; it also suppressed STAT-1 phosphorylation and the nuclear translocation of NF-kappaB in activated keratinocytes. These results suggest that Bakuchicin attenuated AD symptoms, thus suggesting it as a potential therapeutic agent for the treatment of AD.
Osthenol, a prenylated coumarin, as a monoamine oxidase A inhibitor with high selectivity.[Pubmed:30686752]
Bioorg Med Chem Lett. 2019 Mar 15;29(6):839-843.
Osthenol (6), a prenylated coumarin isolated from the dried roots of Angelica pubescens, potently and selectively inhibited recombinant human monoamine oxidase-A (hMAO-A) with an IC50 value of 0.74microM and showed a high selectivity index (SI>81.1) for hMAO-A versus hMAO-B. Compound 6 was a reversible competitive hMAO-A inhibitor (Ki=0.26microM) with a potency greater than toloxatone (IC50=0.93microM), a marketed drug. Isopsoralen (3) and Bakuchicin (1), furanocoumarin derivatives isolated from Psoralea corylifolia L., showed slightly higher IC50 values (0.88 and 1.78microM, respectively) for hMAO-A than 6, but had low SI values (3.1 for both). Other coumarins tested did not effectively inhibit hMAO-A or hMAO-B. A structural comparison suggested that the 8-(3,3-dimethylallyl) group of 6 increased its inhibitory activity against hMAO-A compared with the 6-methoxy group of scopoletin (4). Molecular docking simulations revealed that the binding affinity of 6 for hMAO-A (-8.5kcal/mol) was greater than that for hMAO-B (-5.6kcal/mol) and that of 4 for hMAO-A (-7.3kcal/mol). Docking simulations also implied that 6 interacted with hMAO-A at Phe208 and with hMAO-B at Ile199 by carbon hydrogen bondings. Our findings suggest that osthenol, derived from natural products, is a selective and potent reversible inhibitor of MAO-A, and can be regarded a potential lead compound for the design of novel reversible MAO-A inhibitors.
[Chemical constituents from root of Angelica decursiva].[Pubmed:29139270]
Zhongguo Zhong Yao Za Zhi. 2017 Aug;42(15):2999-3003.
The compounds were isolated and purified by HP20 macroporous adsorption resin, ODS, silica gel, and Sephadex LH-20 column chromatography, as well as semi-preparative HPLC chromatography from the 80% ethanol extract of the root of Angelica decursiva, and their structures were identified based on their physiochemical properties and spectroscopic data. Twelve compounds were structures were identified as (9R,10R)-9-acetoxy-8,8-dimethyl-9,10-dihydro-2H,8H-benzo[1,2-b3,4-b']dipyran-2-on e-10-yl ester (1), Bakuchicin (2), (3', S,4'S)-disenecioyloxy-3',4'-dihydroseselin (3), (3'R,4'R)-3'-angeloyloxy-4'-senecioyloxy-3',4'-dihydroseselincalipteryxin (4), (+)-8,9-dihydro-8-(2-hydroxypropan-2-yl)-2-oxo-2H-furo[2,3h]chromen-9-yl-3-methyl but-2-enoate (5), libanoridin (6), selinidin (7), suberosin (8), crocatone (9), peujaponisinol B (10), peujaponisinol A (11), and ostenol (12), respectively. Compounds 1-5 were isolated from the plants of Angelica genus for the first time. Compounds 7-12 were isolated from A. decursiva for the first time.
Investigation of pharmacokinetic parameters of bakuchicin isolated from Psoralea corylifolia in mice.[Pubmed:28602940]
Fitoterapia. 2017 Jul;120:194-198.
Bakuchicin is a furanocoumarin isolated from the seeds of Psoralea corylifolia, which is used in oriental medicine. However, limited information on the pharmacokinetics of Bakuchicin is available and in addition, no determined method has been devised to quantify Bakuchicin levels in the plasma. In the present study, we developed and validated a quantification method using liquid chromatography (LC) coupled with tandem mass spectrometry (LC-MS/MS), which was applied to a pharmacokinetic investigation in mouse plasma. LC was performed using an ACE 5 C18 column, and a mixture of acetonitrile and water containing 0.1% formic acid was used as the mobile phase at a flow rate of 220muL/min. Bakuchicin transition ions in multiple reaction-monitoring modes using positive ionization were observed at m/z 187.0 to m/z 131.0. Bakuchicin and the internal standard (reserpine) had retention times of 4.5 and 4.3min, respectively. Acceptable linearity (r(2)=0.996) was observed over the concentration range of 20-1000ng/mL, with a lower quantification limit of 20ng/mL in mouse plasma. This method was successfully applied to determine the pharmacokinetic parameters of Bakuchicin in mouse plasma and showed that the bioavailability of Bakuchicin was 58.3% at 5mg/kg oral administration.
Selective Inhibition of Bakuchicin Isolated from Psoralea corylifolia on CYP1A in Human Liver Microsomes.[Pubmed:26977174]
Evid Based Complement Alternat Med. 2016;2016:5198743.
Bakuchicin is a furanocoumarin isolated from Psoralea corylifolia and shows several biological activities. Although there have been studies on the biological effects of Bakuchicin, its modulation potency of CYP activities has not been previously investigated. Here, we investigated the inhibitory effects of Bakuchicin on the activities of CYP isoforms by using a cocktail of probe substrates in pooled human liver microsomes (HLMs) and human recombinant cDNA-expressed CYP. Bakuchicin strongly inhibited CYP1A-mediated phenacetin O-deethylation with an IC50 value of 0.43 muM in HLMs. It was confirmed by human recombinant cDNA-expressed CYP1A1 and CYP1A2 with a K i value of 0.11 muM and 0.32 muM, respectively. A Lineweaver-Burk plot indicated that the inhibition mechanism of Bakuchicin was competitive inhibition. Overall, this is the first study to investigate the potential CYP1A1 and CYP1A2 inhibition associated with Bakuchicin and to report its competitive inhibitory effects on HLMs.
Bakuchicin induces vascular relaxation via endothelium-dependent NO-cGMP signaling.[Pubmed:21442677]
Phytother Res. 2011 Oct;25(10):1574-8.
Bakuchicin is a furanocoumarin derived from the seeds of Psoralea corylifolia. The aim of the present study was to investigate the effect of Bakuchicin on vascular tone in rat aortic tissue. Bakuchicin induced a dose-dependent relaxation of phenylephrine-precontracted rat aorta which was abolished by removal of the endothelium. Pretreatment of the endothelium-intact aortic tissues with NG-nitro-L-arginine methylester (L-NAME) or 1H-[1,2,4]-oxadiazole-[4,3-alpha]-quinoxalin-1-one (ODQ) significantly inhibited the vascular relaxation induced by Bakuchicin. Incubation with Bakuchicin increased the production of cGMP in a concentration-dependent manner, and this effect was blocked by pretreatment with both L-NAME and ODQ. Vascular relaxation induced by Bakuchicin was significantly inhibited by pretreatment with verapamil and diltiazem, but not by several other inhibitors including tetraethylammonium (TEA), glibenclamide, indomethacin, atropine or propranolol. These results suggested that Bakuchicin-induced vasodilatation is closely associated with the endothelium-dependent nitric oxide (NO)/cGMP signaling pathway, with the possible involvement of L-type Ca(2+) channels.
Antibacterial compounds from the seeds of Psoralea corylifolia.[Pubmed:15030932]
Fitoterapia. 2004 Mar;75(2):228-30.
Psoralidin, Bakuchicin, psoralin and angelicin, isolated from the seeds of Psoralea corylifolia, showed significant antibacterial activities against a number of Gram (+) and Gram (-) bacteria.
Bakuchiol: a hepatoprotective compound of Psoralea corylifolia on tacrine-induced cytotoxicity in Hep G2 cells.[Pubmed:11731920]
Planta Med. 2001 Nov;67(8):750-1.
Bioassay-guided fractionation of the H(2)O extract of the seeds of Psoralea corylifolia furnished one hepatoprotective compound, bakuchiol (1), together with two moderately active compounds, Bakuchicin (2) and psoralen (3), on tacrine-induced cytotoxicity in human liver-derived Hep G2 cells. The EC(50) values of compounds 1 - 3 are 1.0, 47.0, 50.0 microg/ml, respectively. Silymarin as a positive control showed the EC(50) value with 5.0 microg/ml.
DNA polymerase and topoisomerase II inhibitors from Psoralea corylifolia.[Pubmed:9544566]
J Nat Prod. 1998 Mar;61(3):362-6.
An ethanol extract of Psoralea corylifolia caused strong DNA polymerase inhibition in a whole cell bioassay specific for inhibitors of DNA replication enzymes. Bioassay-directed purification of the active compounds led to the isolation of the new compound corylifolin (1) and the known compound bakuchiol (2) as DNA polymerase inhibitors. On the basis of the structures of 1 and 2, resveratrol (3) was tested and found to be active as a DNA polymerase inhibitor in this bioassay. Neobavaisoflavone (4) was isolated as a DNA polymerase inhibitor, daidzein (5) as a DNA polymerase and topoisomerase II inhibitor, and Bakuchicin (6) as a topoisomerase II inhibitor.


