BocconolineCAS# 32906-88-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 32906-88-0 | SDF | Download SDF |
| PubChem ID | 181121 | Appearance | Powder |
| Formula | C22H21NO5 | M.Wt | 379.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
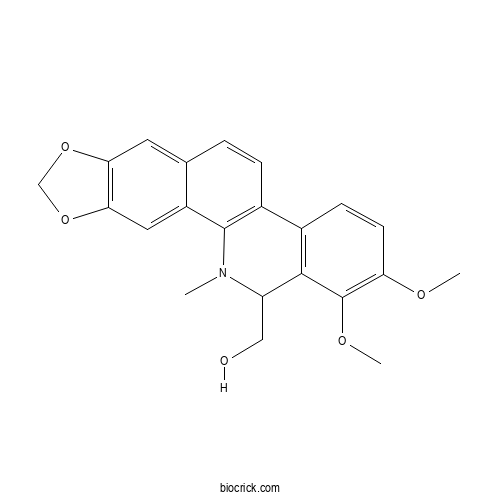
| Chemical Name | (1,2-dimethoxy-12-methyl-13H-[1,3]benzodioxolo[5,6-c]phenanthridin-13-yl)methanol | ||
| SMILES | CN1C(C2=C(C=CC(=C2OC)OC)C3=C1C4=CC5=C(C=C4C=C3)OCO5)CO | ||
| Standard InChIKey | GKBDCSXIKLSKMH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H21NO5/c1-23-16(10-24)20-13(6-7-17(25-2)22(20)26-3)14-5-4-12-8-18-19(28-11-27-18)9-15(12)21(14)23/h4-9,16,24H,10-11H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Bocconoline Dilution Calculator

Bocconoline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6357 mL | 13.1787 mL | 26.3574 mL | 52.7148 mL | 65.8935 mL |
| 5 mM | 0.5271 mL | 2.6357 mL | 5.2715 mL | 10.543 mL | 13.1787 mL |
| 10 mM | 0.2636 mL | 1.3179 mL | 2.6357 mL | 5.2715 mL | 6.5894 mL |
| 50 mM | 0.0527 mL | 0.2636 mL | 0.5271 mL | 1.0543 mL | 1.3179 mL |
| 100 mM | 0.0264 mL | 0.1318 mL | 0.2636 mL | 0.5271 mL | 0.6589 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isosilychristin
Catalog No.:BCN0733
CAS No.:77182-66-2
- Hydroxytyrosol 1-O-glucoside
Catalog No.:BCN0732
CAS No.:76873-99-9
- Wallicoside
Catalog No.:BCN0731
CAS No.:88797-59-5
- Securisteroside
Catalog No.:BCN0730
CAS No.:54964-57-7
- Byzantionoside B
Catalog No.:BCN0729
CAS No.:189109-45-3
- Grasshopper ketone
Catalog No.:BCN0728
CAS No.:41703-38-2
- Cannabisin E
Catalog No.:BCN0727
CAS No.:
- Bisdemethoxyboehmenan
Catalog No.:BCN0726
CAS No.:146918-26-5
- 6-Hydroxyisosativan
Catalog No.:BCN0725
CAS No.:2172624-69-8
- Erythrinin F
Catalog No.:BCN0724
CAS No.:1616592-60-9
- Derrisisoflavone I
Catalog No.:BCN0723
CAS No.:2172624-66-5
- Derrisisoflavone K
Catalog No.:BCN0722
CAS No.:2172624-68-7
- 3-O-Methylellagic acid 3'-O-alpha-rhamnopyranoside
Catalog No.:BCN0735
CAS No.:352280-34-3
- Yinxiancaoside C
Catalog No.:BCN0736
CAS No.:1159908-74-3
- Methylpicraquassioside B
Catalog No.:BCN0737
CAS No.:1443757-89-8
- Methyl 2-hydroxy-3,4-dimethoxybenzoate
Catalog No.:BCN0738
CAS No.:6395-23-9
- 3'-O-Methylviolanone
Catalog No.:BCN0739
CAS No.:56973-42-3
- Lariciresinol 4-O-glucoside
Catalog No.:BCN0740
CAS No.:143663-00-7
- 10-O-trans-p-coumaroylscandoside
Catalog No.:BCN0741
CAS No.:870785-25-4
- Cuneataside C
Catalog No.:BCN0742
CAS No.:871720-16-0
- 5,6,7,3',4',5'-Hexamethoxyflavanone
Catalog No.:BCN0743
CAS No.:74064-17-8
- Theaflavanoside II
Catalog No.:BCN0744
CAS No.:943785-09-9
- 3',8-Dihydroxyvestitol
Catalog No.:BCN0745
CAS No.:122587-87-5
- Sativanone
Catalog No.:BCN0746
CAS No.:70561-31-8
Active constituents of Zanthoxylum nitidium from Yunnan Province against leukaemia cells in vitro.[Pubmed:34301301]
BMC Chem. 2021 Jul 23;15(1):44.
Zanthoxylum nitidium (Roxb.) DC (Rutaceae) is well known for inhibiting the proliferation of human gastric, liver, kidney and lung cancer cells, though research on its potential use in treating leukaemia is relatively rare. Twenty-six compounds were isolated from the chloroform and petroleum ether extracts of the roots and leaves of Z. nitidium (Zanthoxylum nitidium). They were ( +)-9'-O-transferuloyl-5, 5'-dimethoxylaricriresinol (1), 8-(3'-oxobut-1'-en-1'-yl)-5, 7-dimethoxy-coumarin (2), 5, 7, 8-trimethoxy-coumarin (3), 5-(3', 3'-dimethyl-2'-butenyloxy)-7, 8-dimethoxy-coumarin (4), 2-(5-methoxy-2-methyl-1H-indol-3-yl) methyl acetate (5), 2'-(5, 6-dihydrochleletrythrine-6-yl) ethyl acetate (6), 6-acetonyldi-hydrochelerythrine (7), 6beta-hydroxymethyldihydronitidine (8), Bocconoline (9), zanthoxyline (10), O-methylzanthoxyline (11), rhoifoline B (12), N-nornitidine (13), nitidine (14), chelerythrine (15), 4-hydroxyl-7,8-dimethoxy-furoquinoline (16), dictamnine (17), gamma-fagarine (18), skimmianine (19), robustine (20), R-( +)-platydesmine (21), 4-methoxyl-1-methyl-2-quinoline (22), 4-methoxy-2-quinolone (23), liriodenine (24), aurantiamide acetate (25), 10-O-demethyl-12-O-methylarnottianamide (26). Four among them, compounds 4 - 6 and 16, were first confirmed in this study by UV, IR, 1D, 2D NMR and HR-ESI-MS spectra. Compounds 1 - 2 and 11 were isolated from Z. nitidium for the first time. Of the assayed compounds, 1, 2, 9, 10, 14, 15 and 24, exhibited good inhibitory activities in the leukaemia cell line HEL, whereas compound 14 (IC50: 3.59 microM) and compound 24 (IC50: 15.95 microM) exhibited potent inhibitory activities. So, to further investigate the possible mechanisms, cell cycle and apoptosis assays were performed, which indicated that compound 14 causes obvious S-phase arrest in HEL cells and induced apoptosis, whereas compound 24 only induced apoptosis. The present results suggested both compounds 14 and 24 are promising potential anti-leukaemia drug candidates.
Chemical constituents from Macleaya cordata (Willd) R. Br. and their phenotypic functions against a Parkinson's disease patient-derived cell line.[Pubmed:33065438]
Bioorg Med Chem. 2020 Nov 1;28(21):115732.
Cytological profiling (CP) assay against a human olfactory neuroshpere-derived (hONS) cell line using a library of traditional Chinese medicinal plant extracts gave indications that the ethanolic extract of Macleaya cordata (Willd) R. Br. elicited strong perturbations to various cellular components. Further chemical investigation of this extract resulted in the isolation of two new benzo[c]phenanthridine alkaloids, (6R)-10-methoxyBocconoline (1) and 6-(1-hydroxyethyl)-10-methoxy-5,6-dihydrochelerythrine (2). Their planar structures were elucidated by extensive 1D and 2D NMR studies, together with MS data. The absolute configuration for position C-6 of 1 and relative configurations for position C-6 and C-1' of 2 were assigned by density functional theory (DFT) calculations of ECD and NMR data, respectively. Also isolated were fourteen known metabolites, including ten alkaloids (3-12) and four coumaroyl-containing compounds (13-16). Cytological profiling of the isolates against Parkinson's Disease (PD) patient-derived olfactory cells revealed Bocconoline (3) and 6-(1-hydroxyethyl)-5,6-dihydrochelerythrine (4) significantly perturbated the features of cellular organelles including early endosomes, mitochondria and autophagosomes. Given that hONS cells from PD patients model some functional aspects of the disease, the results suggested that these phenotypic profiles may have a role in the mechanisms underlying PD and signified the efficacy of CP in finding potential chemical tools to study the biological pathways in PD.
Identification and quantification of the main active anticancer alkaloids from the root of Glaucium flavum.[Pubmed:24317429]
Int J Mol Sci. 2013 Dec 2;14(12):23533-44.
Glaucium flavum is used in Algerian folk medicine to remove warts (benign tumors). Its local appellations are Cheqiq el-asfar and Qarn el-djedyane. We have recently reported the anti-tumoral activity of Glaucium flavum root alkaloid extract against human cancer cells, in vitro and in vivo. The principal identified alkaloid in the extract was protopine. This study aims to determine which component(s) of Glaucium flavum root extract might possess potent antitumor activity on human cancer cells. Quantitative estimation of Glaucium flavum alkaloids was realized by HPLC-DAD. Glaucium flavum effect on human normal and cancer cell viability was determined using WST-1 assay. Quantification of alkaloids in Glaucium flavum revealed that the dried root part contained 0.84% of protopine and 0.07% of Bocconoline (w/w), while the dried aerial part contained only 0.08% of protopine, glaucine as the main alkaloid, and no Bocconoline. In vitro evaluation of the growth inhibitory activity on breast cancer and normal cells demonstrated that purified protopine did not reproduce the full cytotoxic activity of the alkaloid root extract on cancer cell lines. On the other hand, Bocconoline inhibited strongly the viability of cancer cells with an IC50 of 7.8 microM and only a low cytotoxic effect was observed against normal human cells. Our results showed for the first time that protopine is the major root alkaloid of Glaucium flavum. Finally, we are the first to demonstrate a specific anticancer effect of Glaucium flavum root extract against breast cancer cells, which can be attributed, at least in part, to Bocconoline.
Further alkaloids from bark of Fagara mayu.[Pubmed:17404954]
Planta Med. 1983 Jun;48(2):77-80.
In addition to the alkaloids canthin-6-one; dictamnine, chelerythrine, gamma-fagarine, skimmianine, magnoflorine and 11-(2'-ketobutane)-dihydrochelerythrine, four further alkaloids were isolated from the bark of Fagara mayu (Bert. ex Hook et Arn.) Engler and edulinine, Bocconoline, tembetarine were identified. A new base, N-methylpseudolaudanine, was also isolated.


