SativanoneCAS# 70561-31-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 70561-31-8 | SDF | Download SDF |
| PubChem ID | 13886678 | Appearance | Powder |
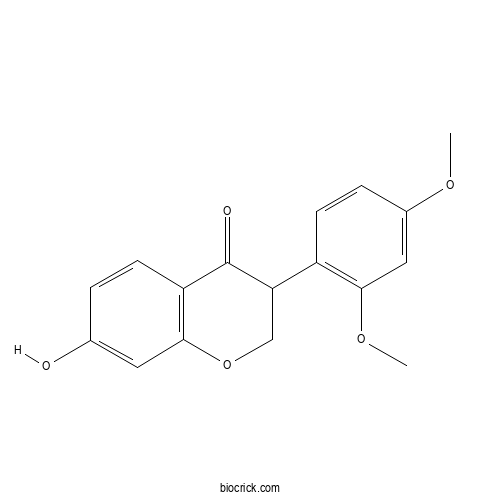
| Formula | C17H16O5 | M.Wt | 300.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-(2,4-dimethoxyphenyl)-7-hydroxy-2,3-dihydrochromen-4-one | ||
| SMILES | COC1=CC(=C(C=C1)C2COC3=C(C2=O)C=CC(=C3)O)OC | ||
| Standard InChIKey | JOVYBWHPTQRVNZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H16O5/c1-20-11-4-6-12(15(8-11)21-2)14-9-22-16-7-10(18)3-5-13(16)17(14)19/h3-8,14,18H,9H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Sativanone Dilution Calculator

Sativanone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.33 mL | 16.65 mL | 33.3 mL | 66.6001 mL | 83.2501 mL |
| 5 mM | 0.666 mL | 3.33 mL | 6.66 mL | 13.32 mL | 16.65 mL |
| 10 mM | 0.333 mL | 1.665 mL | 3.33 mL | 6.66 mL | 8.325 mL |
| 50 mM | 0.0666 mL | 0.333 mL | 0.666 mL | 1.332 mL | 1.665 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.333 mL | 0.666 mL | 0.8325 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3',8-Dihydroxyvestitol
Catalog No.:BCN0745
CAS No.:122587-87-5
- Theaflavanoside II
Catalog No.:BCN0744
CAS No.:943785-09-9
- 5,6,7,3',4',5'-Hexamethoxyflavanone
Catalog No.:BCN0743
CAS No.:74064-17-8
- Cuneataside C
Catalog No.:BCN0742
CAS No.:871720-16-0
- 10-O-trans-p-coumaroylscandoside
Catalog No.:BCN0741
CAS No.:870785-25-4
- Lariciresinol 4-O-glucoside
Catalog No.:BCN0740
CAS No.:143663-00-7
- 3'-O-Methylviolanone
Catalog No.:BCN0739
CAS No.:56973-42-3
- Methyl 2-hydroxy-3,4-dimethoxybenzoate
Catalog No.:BCN0738
CAS No.:6395-23-9
- Methylpicraquassioside B
Catalog No.:BCN0737
CAS No.:1443757-89-8
- Yinxiancaoside C
Catalog No.:BCN0736
CAS No.:1159908-74-3
- 3-O-Methylellagic acid 3'-O-alpha-rhamnopyranoside
Catalog No.:BCN0735
CAS No.:352280-34-3
- Bocconoline
Catalog No.:BCN0734
CAS No.:32906-88-0
- 10-O-trans-p-Feruloylscandoside
Catalog No.:BCN0747
CAS No.:1428268-72-7
- 2,3-Dehydrosilychristin
Catalog No.:BCN0748
CAS No.:57499-41-9
- Picraquassioside B
Catalog No.:BCN0749
CAS No.:169312-05-4
- Mucronulatol
Catalog No.:BCN0750
CAS No.:20878-98-2
- Tortoside B (Manglieside E)
Catalog No.:BCN0751
CAS No.:190655-17-5
- Caffeic acid 4-O-glucuronide
Catalog No.:BCN0752
CAS No.:1093679-71-0
- Ternstroside C
Catalog No.:BCN0760
CAS No.:914649-21-1
- Ternstroside A
Catalog No.:BCN0761
CAS No.:914649-13-1
- Ternstroside B
Catalog No.:BCN0762
CAS No.:914649-16-4
- Ternstroside D
Catalog No.:BCN0764
CAS No.:914649-26-6
- Ternstroside E
Catalog No.:BCN0765
CAS No.:914649-31-3
- Ternstroside F
Catalog No.:BCN0766
CAS No.:914649-36-8
Integration of lipidomics and metabolomics for the authentication of camellia oil by ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry coupled with chemometrics.[Pubmed:34801288]
Food Chem. 2022 Mar 30;373(Pt B):131534.
The integration of lipidomics and metabolomics approaches, based on UPLC-QTOF-MS technology coupled with chemometrics, was established to authenticate camellia oil adulterated with rapeseed oil, peanut oil, and soybean oil. Lipidomics revealed that the glyceride profile provides a prospective authentication of camellia oil, but no characteristic markers were available. Sixteen characteristic markers were identified by metabolomics. For camellia oil, all six markers were sapogenins of oleanane-type triterpene saponins. Lariciresinol, sinapic acid, doxercalciferol, and an unknown compound were identified as markers for rapeseed oil. Characteristic markers in peanut oil were formononetin, Sativanone, and medicarpin. In the case of soybean oil, the characteristic markers were dimethoxyflavone, daidzein, and genistein. The established OPLS-DA and OPLS prediction models were highly accurate in the qualitative and quantitative analyses of camellia oil adulterated with 5% other oils. These results indicate that the integration of lipidomics and metabolomics approaches has great potential for the authentication of edible oils.
Dihydroisocoumarins and Dihydroisoflavones from the Rhizomes of Dioscorea collettii with Cytotoxic Activity and Structural Revision of 2,2'-Oxybis(1,4-di-tert-butylbenzene).[Pubmed:34500814]
Molecules. 2021 Sep 4;26(17). pii: molecules26175381.
The investigation of the constituents of the rhizomes of Dioscorea collettii afforded one new dihydroisocoumarin, named (-)-montroumarin (1a), along with five known compounds-montroumarin (1b), 1,1'-oxybis(2,4-di-tert-butylbenzene) (2), (3R)-3'-O-methylviolanone (3a), (3S)-3'-O-methylviolanone (3b), and (RS)-Sativanone (4). Their structures were elucidated using extensive spectroscopic methods. To the best of our knowledge, compound 1a is a new enantiomer of compound 1b. The NMR data of compound 2 had been reported but its structure was erroneous. The structure of compound 2 was revised on the basis of a reinterpretation of its NMR data (1D and 2D) and the assignment of the (1)H and (13)C NMR data was given rightly for the first time. Compounds 3a-4, three dihydroisoflavones, were reported from the Dioscoreaceae family for the first time. The cytotoxic activities of all the compounds were tested against the NCI-H460 cell line. Two dihydroisocoumarins, compounds 1a and 1b, displayed moderate cytotoxic activities, while the other compounds showed no cytotoxicity.
Qualitative and Quantitative Phytochemical Analysis of Ononis Hairy Root Cultures.[Pubmed:33584762]
Front Plant Sci. 2021 Jan 13;11:622585.
Hairy root cultures are genetically and biochemically stable, and they regularly possess the same or better biosynthetic capabilities for specialized (secondary) metabolite production compared to the intact plant. Ononis species are well-known herbal remedies in ethnopharmacology and rich sources of isoflavonoids. Besides isoflavones, less prevalent isoflavones and pterocarpans with valuable biological effects can be found in Ononis species as well. As these plants are only collected but not cultivated, biotechnological methods could play a role in the larger-scale extraction of Ononis isoflavonoids. Regarding this information, we aimed to establish Ononis spinosa and Ononis arvensis hairy root cultures (HRCs) and analyze the isoflavonoid profile of hairy root cultures qualitatively and quantitatively, in order to define their capacity to produce biologically valuable isoflavonoids. During the qualitative description, beside isoflavonoids, two new phenolic lactones, namely, bulatlactone 2''-O-beta-D-glucoside and ononilactone, were isolated, and their structures were characterized for the first time. Altogether, 29 compounds were identified by the means of UPLC-Orbitrap-MS/MS. Based on UHPLC-UV-DAD measurements, the isoflavonoid spectrum of the Ononis HRCs differed markedly from wild-grown samples, as they produce a limited range of the scaffolds. The most abundant compounds in the HRCs were medicarpin glucoside and Sativanone glucoside. The overall isoflavonoid production of the cultures was comparable to wild-grown O. arvensis and approximately twice as high as in wild-grown O. spinosa samples. As the overall content of wild-grown samples include more isoflavonoid derivatives, the HRCs contain structurally less divergent isoflavonoids but in higher quantity.
[A new cytotoxic isoflavane from Dalbergiae Odoriferae Lignum].[Pubmed:32495561]
Zhongguo Zhong Yao Za Zhi. 2020 May;45(9):2122-2129.
Fourteen compounds were isolated from the ethanol extract of Dalbergiae Odoriferae Lignum by various chromatographic techniques, including column chromatographies on silica gel, Sephadex LH-20 and semi-preparative HPLC. Their structures were determined by spectroscopic techniques as S-3'-hydroxy-7,2',4'-trimethoxyisoxane(1), 2-(2',4'-dimethoxyphenyl)-6-hydroxybenzofuran(2), 2-(2'-hydroxy-4'-methoxyphenyl)-6-methoxybenzofuran(3), 7,2',4'-trimethoxydihydroisoflavone(4), Sativanone(5), 3,9-dimethoxy-6H-benzofuro[3,2-c]chromen-6-one(6),(6 aS,11 aS)-homopterocarpin(7),(6 aS,11 aS)-8-hydroxy-3,9-dimethoxypterocarpan(8),(6 aS,11 aS)-3,8,9-trimethoxypterocarpan(9), isodalbergin(10), isoliquiritigenin(11), butein(12), butin(13) and 3,7,4'-trihydroxyflavone(14). Among them, compound 1 was a new compound, while 2 and 3 were new natural products, 6, 8, 9 and 14 were isolated for the first time from Dalbergiae Odoriferae Lignum. Compounds 1-14 were tested for their cytotoxic activity against human hepatoma cell line BEL-7402, human gastric cancer cell line SCG-7901, human lung cancer cell line A549, human chronic myeloid leukemia cell line K562 and HeLa human cervical cancer cellline by MTT method. Compound 1 exhibited significant cytotoxicity with IC_(50) values ranging from 2.85 to 11.62 mug.mL~(-1). In addition, 2, 11 and 12 showed weak cytotoxic activities.
Analysis of Flavonoids in Dalbergia odorifera by Ultra-Performance Liquid Chromatography with Tandem Mass Spectrometry.[Pubmed:31963485]
Molecules. 2020 Jan 17;25(2). pii: molecules25020389.
Dalbergia odorifera, a traditional Chinese medicine, has been used to treat cardio- and cerebrovascular diseases in China for thousands of years. Flavonoids are major active compounds in D. odorifera. In this paper, a rapid and sensitive ultra-high performance liquid chromatography-triple quadrupole mass spectrometry method was developed and validated for simultaneous determination of 17 flavonoids in D. odorifera. Quantification was performed by multiple reaction monitoring using electrospray ionization in negative ion mode. Under the optimum conditions, calibration curves for the 17 analytes displayed good linearity (r(2) > 0.9980). The intra- and inter-day precisions (relative standard deviations) were lower than 5.0%. The limit of quantitation ranged from 0.256 to 18.840 ng/mL. The mean recovery range at three spiked concentrations was 94.18-101.97%. The validated approach was successfully applied to 18 samples of D. odorifera. Large variation was observed for the contents of the 17 analytes. Sativanone and 3'-O-methylviolanone were the dominant compounds. The fragmentation behaviors of six flavonoids were investigated using UPLC with quadrupole time-of-flight tandem mass spectrometry. In negative ion electrospray ionization mass spectrometry, all the flavonoids yielded prominent [M - H](-) ions. Fragments for losses of CH3, CO, and CO2 were observed in the mass spectra. Formononetin, liquiritigenin, isoliquiritigenin, Sativanone, and alpinetin underwent retro-Diels-Alder fragmentations. The proposed method will be helpful for quality control of D. odorifera.
Isoflavonoids with inhibiting effects on human hyaluronidase-1 and norneolignan clitorienolactone B from Ononis spinosa L. root extract.[Pubmed:30176279]
Fitoterapia. 2018 Oct;130:169-174.
Human hyaluronidase-1 (Hyal-1) is one of the main enzymes in the homeostasis of hyaluronic acid (HA), the main polysaccharide of extracellular matrix. Development of specific Hyal-1 inhibitors might be a promising target for improved wound healing, tissue regeneration, and looking at renal function for diuresis. By using surface-displayed Hyal-1 on Escherichia coli F470 cells, HA as substrate and stains-all method for quantification of undegraded HA, the respective enzyme activity can be determined easily. Based on the traditional use of extracts from the roots from Ononis spinosa L. (Restharrow root) as a weak diuretic to achieve flushing of the urinary tract and as an adjuvant in minor urinary complaints the herbal material was selected for bioactivity guided fractionation for compounds with Hyal-1 inhibition activity. Hot water and hydroalcoholic extracts showed moderate inhibiting effects (IC50 1.36 resp. 0.73mg/mL) while dichloromethane extract exerted an IC50 of 190mug/mL. Bioassay guided fractionation of the dichloromethane extract yielded four isoflavonoids with anti Hyal-1 activity: onogenin 1, Sativanone 2, medicarpin 3 and calycosin-D 4 with inhibition rates of 25.4, 61.2, 22.4 and 23.0%, respectively at test concentration level of 250muM. The norneolignan clitorienolactone B 5, the first time described for the genus Ononis, was inactive. The IC50 of Sativanone, the most active compound was determined with 1501muM, which was better than that of the positive control glycyrrhizinic acid (177muM). Thus, a possible explanation for diuretic properties of Ononis spinosa L. root extract may be postulated from the results so far obtained.
New Records of Potent In-Vitro Antidiabetic Properties of Dalbergia tonkinensis Heartwood and the Bioactivity-Guided Isolation of Active Compounds.[Pubmed:29966279]
Molecules. 2018 Jun 29;23(7). pii: molecules23071589.
Alpha-glucosidase inhibitory activity has been commonly used for the evaluation of antidiabetic property in vitro. The aim of this study is to investigate and characterize Dalbergia tonkinensis as a potential source of antidiabetic compounds. The screening of the active parts used, such as trunk bark, heartwood, and the leaves of Dalbergia tonkinensis indicated that all these extracted parts used with methanol demonstrated potent alpha-glucosidase inhibitory activity. The in vitro antidiabetic property of Dalbergia tonkinensis was notably recorded for the first time and showed activity (EC50 = 0.17(-)0.78 mg/mL) comparable to those of reported potent herbal extracts (EC50 = 0.25(-)4.0 mg/mL) and higher activity than that of acarbose, a commercial antidiabetic drug (EC50 = 1.21 mg/mL). The stability tests revealed that the heartwood of Dalbergia tonkinensis extract (HDT) possesses high pH stability with relative activity in the range of 80(-)98%. Further bioassay-guided purification led to the isolation of 2 active compounds identified as Sativanone and formononetin from the ethyl acetate fraction and water fraction of HDT, respectively. These alpha-glucosidase inhibitors (aGIs) show promising inhibition against various types of alpha-glucosidases. Remarkably, these inhibitors were determined as new mammalian aGIs, showing good effect on rat alpha-glucosidase. The results suggest that Dalbergia tonkinensis is a potent source of aGIs and suggest promise in being developed as functional food with antidiabetic efficacy. The results of this study also enrich our knowledge concerning current biological activity and constituents of Dalbergia tonkinensis species.
Separation and characterization of homopipecolic acid isoflavonoid ester derivatives isolated from Ononis spinosa L. root.[Pubmed:29803686]
J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Aug 1;1091:21-28.
Spiny restharrow root (Ononis spinosa L.) and its preparations are mainly used for the treatment of urinary infections or bladder stones in numerous countries. Spiny restharrow root is rich in isoflavonoids (formononetin, calycosin and pseudobaptigenin), pterocarpans (medicarpin and maackiain) and dihydroisoflavonoids (onogenin and Sativanone), which metabolites are present as glucosides, glucoside malonates, glucoside acetates and free aglycones in the root. The in-depth analysis of tandem mass spectrometric (MS) and high-resolution MS (HR-MS) data revealed the presence of nitrogen-containing compounds in the root extracts. An ion-exchange-based purification and a preparative-scale reversed phase chromatographic isolation procedure was developed for the characterization of these new natural products. For the unambiguous identification of the isolated compounds NMR experiments were carried out. The thorough characterization confirmed the presence of six piperidin-2-yl-acetic acid (homopipecolic acid) esters of isoflavonoid glucosides. This is the first report of homopipecolic acid esters isolated from higher plants.
Isoflavonoids as wound healing agents from Ononidis Radix.[Pubmed:28989011]
J Ethnopharmacol. 2018 Jan 30;211:384-393.
ETHNOPHARMACOLOGICAL RELEVANCE: Dried roots of Ononis spinosa L. are traditionally used for their diuretic, anti-inflammatory and wound healing effects. AIM OF THE STUDY: Isolation of the bioactive compounds of Ononis spinosa L. subsp. leiosperma (Boiss.) Sirj. MATERIALS AND METHODS: Ethyl acetate extract prepared from the roots of Ononis spinosa L. subsp. leiosperma (Boiss.) Sirj. was subjected to silica gel column. The fractions were tested for their wound healing and anti-inflammatory activities. Linear incision and circular excision wound models and hydroxypyroline estimation assay were used for the wound healing activity. Carrageenan-induced hind paw edema, TPA-induced ear edema and acetic acid-induced increase in capillary permeability tests as acute inflammation; FCA-induced arthritis as chronic inflammation models were used for the assessment of anti-inflammatory activity. Antioxidant capacities of the fractions were tested using 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, 2,2-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid (ABTS) scavenging activity assay, reducing power assay and hydroxyl radical (OH(-)) scavenging assay. The isolation procedure was continued with the active fraction (Fr-E5). RESULTS: Fr-E5 exhibited remarkable wound healing activity with the 33.4% tensile strength value on the linear incision wound model and 51.4% reduction of the wound area at the day 12 on the circular excision wound model. Hydroxyproline content of the tissue treated by Fr-E5 was found to be 30.9 +/- 0.72mug/mg. Acetic acid induced increase in capillary permeability test results revealed that Fr-E5 inhibited inflammation by the value of 40.3%. Fr-E5 showed 28.1-32.2% inhibition in carrageenan-induced hind paw edema test while did not possess activity on TPA-induced ear edema and FCA-induced arthritis models. Trifolirhizin, ononin, medicarpin-3-O-glucoside, onogenin-7-O-glucoside and Sativanone-7-O-glucoside were isolated from Fr-E5 and tested for their wound healing activities using by measuring their inhibition of hyaluronidase, collagenase and elastase enzymes. Ononin and Sativanone-7-O-glucoside inhibited hyaluronidase and elastase enzymes by 31.66% and 41.75%; 45.58% and 46.88% values respectively at the dose of 100mug/mL. CONCLUSION: Among five isolated compounds, ononin and Sativanone-7-O-glucoside were found to inhibit hyaluronidase and elastase enzymes. According to the results, these compounds may majorly be responsible for the wound healing activity of the extract.
Characterization and identification of isoflavonoid glycosides in the root of Spiny restharrow (Ononis spinosa L.) by HPLC-QTOF-MS, HPLC-MS/MS and NMR.[Pubmed:26874257]
J Pharm Biomed Anal. 2016 May 10;123:74-81.
Restharrow root has been used in traditional medicine for thousands of years; however, the active ingredients responsible for the diuretic effect are still unknown. Previous studies have proved that the root extract contains isoflavonoids, however only few derivatives were identified, mostly relying on retention times or UV data. The aim of our work was to perform a detailed structural characterization of the complete isoflavonoid profile in the aqueous-methanolic extract of Ononis spinosa root by high-performance liquid chromatography coupled with electrospray ionization accurate-mass quadrupole time-of-flight and tandem mass spectrometry in positive ionization mode (HPLC-ESI-QTOF-MS, HPLC-ESI-MS/MS) and nuclear magnetic resonance spectroscopy (NMR). On the basis of the accurate masses and fragmentation patterns isoflavones (formononetin, calycosin and pseudobaptigenin) and pterocarpans (maackiain and medicarpin) were identified. Two further dihydroisoflavone aglycones, namely onogenin and Sativanone and a unique glucoside were isolated and their structures were elucidated by NMR experiments. Calycosin, onogenin and Sativanone were detected in this plant for the first time. In contrast to previous works, the presence of biochanin A could not be confirmed, however its regioisomer calycosin and its derivatives were identified. Similarly, neither tectorigenin derivatives could be detected, however the isobar compound Sativanone and its various glucosides were elucidated. The presence of genistein and daidzein could not be confirmed in the extract. Fragmentation pathways for onogenin and Sativanone are presented. In the aqueous-methanolic extract 9 glucosides, 6 minor and 8 major glucoside malonates, 4 glucoside acetates and 7 aglycones were found. In total, 34 compounds were successfully identified.
Dalbergia odorifera Extract Ameliorates UVB-Induced Wrinkle Formation by Modulating Expression of Extracellular Matrix Proteins.[Pubmed:25620496]
Drug Dev Res. 2015 Feb;76(1):48-56.
Preclinical Research Emerging evidence suggests that Dalbergia odorifera T. Chen (Leguminosae), an indigenous medicinal herb, has therapeutic potential. This study examined the antiwrinkle effects of ethanol extracts of D. odorifera in UVB-irradiated human skin cells. Ethanol extracts of D. odorifera and thier constituents, dalbergin and Sativanone, induced expression of collagen type I and transforming growth factor (TGF)-beta1 in human dermal fibroblasts. In HR-1 hairless mice exposed to UVB, the ethanol extract reduced wrinkle formation and skin thickness. This inhibitory effect of ethanol extract was associated with the restoration of collagen type I, TGF-beta1, and elastin to levels approaching those in skin tissues not exposed to UVB, which was accompanied by the reduction of matrix metalloproteinase-2 and upregulation of tissue inhibitors of metalloproteinase (TIMP)-2 and TIMP-3 in skin tissue exposed to UVB. These results suggest that the ethanol extracts prevent some effects of photoaging and maintain skin integrity by regulating the degradation of the extracellular matrix proteins. (c) 2015 Wiley Periodicals, Inc.
Ethanol extract of Dalbergia odorifera protects skin keratinocytes against ultraviolet B-induced photoaging by suppressing production of reactive oxygen species.[Pubmed:25560618]
Biosci Biotechnol Biochem. 2015;79(5):760-6.
Dalbergia odorifera T. Chen (Leguminosae), an indigenous medicinal herb, has been widely used in northern and eastern Asia to treat diverse diseases. Here, we investigated the anti-senescent effects of ethanolic extracts of Dalbergia odorifera (EEDO) in ultraviolet (UV) B-irradiated skin cells. EEDO significantly inhibited UVB-induced senescence of human keratinocytes in a concentration-dependent manner, concomitant with inhibition of reactive oxygen species (ROS) generation. UVB-induced increases in the levels of p53 and p21, biomarkers of cellular senescence, were almost completely abolished in the presence of EEDO. Sativanone, a major constituent of EEDO, also attenuated UVB-induced senescence and ROS generation in keratinocytes, indicating that Sativanone is an indexing (marker) molecule for the anti-senescence properties of EEDO. Finally, treatment of EEDO to mice exposed to UVB significantly reduced ROS levels and the number of senescent cells in the skin. Thus, EEDO confers resistance to UVB-induced cellular senescence by inhibiting ROS generation in skin cells.
A stereoselective switch: enantiodivergent approach to the synthesis of isoflavanones.[Pubmed:25314579]
Chemistry. 2014 Nov 17;20(47):15354-9.
A modular six-step asymmetric synthesis of two naturally occurring and three non-natural isoflavanones containing tertiary alpha-aryl carbonyls is reported. This synthetic route, utilising a Pd-catalyzed decarboxylative asymmetric protonation, produces isoflavanones in excellent enantioselectivities from 76-97 %. A switch in the sense of stereoinduction was observed when different H(+) sources were employed, showing the first example of dual stereocontrol in an asymmetric protonation reaction. The first enantioselective synthesis of the naturally occurring isoflavanones Sativanone and 3-o-methylviolanone has been accomplished.
[Anti-inflammation of flavonoid compounds from Dalbergia odorifera T. Chen in lipopolysaccharide stimulated RAW264.7 macrophages].[Pubmed:23837974]
Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2013 Jul;29(7):681-4.
OBJECTIVE: To screen, isolate and identify the compounds with anti-inflammatory activity from Dalbergia odorifera T. Chen. METHODS: Lipopolysaccharide (LPS) was used to stimulate RAW264.7 macrophages, and this model was further used to screen and separate active compounds from Dalbergia odorifera T. Chen, followed by compound chemical structure identification using mass spectrometry and nuclear magnetic resonance. Griess assay was performed to measure the released nitric oxide (NO), and ELISA was applied to detect TNF-alpha content. RESULTS: Compound I (Sativanone) showed strong anti-inflammatory activity (IC50;=12.48 g/mL). Isoliquiritigenin (compound III), naringenin (compound IV) and liquiritigenin (compound V) had moderate anti-inflammatory activity, with IC50; value at 18.33, 42.59, 29.43 g/mL, respectively. Compound II and compound VI had no anti-inflammatory activity with concentration at 50 g/mL. Sativanone, isoliquiritigenin, liquiritigenin and naringenin at their concentrations ranging from 3.125-50 g/mL, could inhibit NO release in RAW264.7 cells stimulated by LPS. Sativanone (compound I) could also inhibit TNF-alpha secretion from LPS stimulated RAW264.7 cells. CONCLUSION: Liquiritigenin, isoliquiritigenin, naringenin and Sativanone isolated from Dalbergia odorifera T. Chen, could reduce NO release from LPS-stimulated macrophage cell line (RAW264.7), at a dose-dependent manner. What's more, Sativanone play an anti-inflammatory role also through inhibiting inflammatory cytokine TNF-alpha.
Antibacterial activity of the flavonoids from Dalbergia odorifera on Ralstonia solanacearum.[Pubmed:22117168]
Molecules. 2011 Nov 25;16(12):9775-82.
Phytohemical investigation on the heartwood of Dalbergia odorifera resulted in the isolation of nine flavonoids. Their structures were elucidated as Sativanone (1), (3R)-vestitone (2), (3R)-2',3',7-trihydroxy-4'-methoxyisoflavanone (3), (3R)-4'-methoxy-2',3,7-trihydroxyisoflavanone (4), carthamidin (5), liquiritigenin (6), isoliquiritigenin (7), (3R)-vestitol (8), and sulfuretin (9) based on their spectral data. All compounds were evaluated for their inhibitory activity against Ralstonia solanacearum. This is the first report about anti-R. solanacearum activity of the compounds from D. odorifera.


