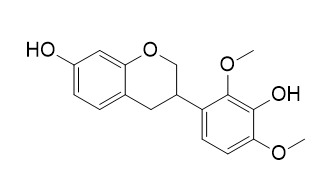
MucronulatolCAS# 20878-98-2 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 20878-98-2 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C17H18O5 | M.Wt | 302.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Mucronulatol Dilution Calculator

Mucronulatol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.308 mL | 16.5399 mL | 33.0797 mL | 66.1594 mL | 82.6993 mL |
| 5 mM | 0.6616 mL | 3.308 mL | 6.6159 mL | 13.2319 mL | 16.5399 mL |
| 10 mM | 0.3308 mL | 1.654 mL | 3.308 mL | 6.6159 mL | 8.2699 mL |
| 50 mM | 0.0662 mL | 0.3308 mL | 0.6616 mL | 1.3232 mL | 1.654 mL |
| 100 mM | 0.0331 mL | 0.1654 mL | 0.3308 mL | 0.6616 mL | 0.827 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Picraquassioside B
Catalog No.:BCN0749
CAS No.:169312-05-4
- 2,3-Dehydrosilychristin
Catalog No.:BCN0748
CAS No.:57499-41-9
- 10-O-trans-p-Feruloylscandoside
Catalog No.:BCN0747
CAS No.:1428268-72-7
- Sativanone
Catalog No.:BCN0746
CAS No.:70561-31-8
- 3',8-Dihydroxyvestitol
Catalog No.:BCN0745
CAS No.:122587-87-5
- Theaflavanoside II
Catalog No.:BCN0744
CAS No.:943785-09-9
- 5,6,7,3',4',5'-Hexamethoxyflavanone
Catalog No.:BCN0743
CAS No.:74064-17-8
- Cuneataside C
Catalog No.:BCN0742
CAS No.:871720-16-0
- 10-O-trans-p-coumaroylscandoside
Catalog No.:BCN0741
CAS No.:870785-25-4
- Lariciresinol 4-O-glucoside
Catalog No.:BCN0740
CAS No.:143663-00-7
- 3'-O-Methylviolanone
Catalog No.:BCN0739
CAS No.:56973-42-3
- Methyl 2-hydroxy-3,4-dimethoxybenzoate
Catalog No.:BCN0738
CAS No.:6395-23-9
- Tortoside B (Manglieside E)
Catalog No.:BCN0751
CAS No.:190655-17-5
- Caffeic acid 4-O-glucuronide
Catalog No.:BCN0752
CAS No.:1093679-71-0
- Ternstroside C
Catalog No.:BCN0760
CAS No.:914649-21-1
- Ternstroside A
Catalog No.:BCN0761
CAS No.:914649-13-1
- Ternstroside B
Catalog No.:BCN0762
CAS No.:914649-16-4
- Ternstroside D
Catalog No.:BCN0764
CAS No.:914649-26-6
- Ternstroside E
Catalog No.:BCN0765
CAS No.:914649-31-3
- Ternstroside F
Catalog No.:BCN0766
CAS No.:914649-36-8
- 5,6,7,3',4'-Pentamethoxyflavanone
Catalog No.:BCN0753
CAS No.:104193-93-3
- Dihydrophaseic acid 4'-O-beta-D-glucopyranoside
Catalog No.:BCN0754
CAS No.:78914-56-4
- Ternatumoside II
Catalog No.:BCN0755
CAS No.:1473419-87-2
- ZP-amide C
Catalog No.:BCN0756
CAS No.:412316-38-2
Machaerium opacum Vogel (Fabaceae): phytochemical study and toxicity to Atta sexdens Linnaeus (Hymenoptera: Formicidae).[Pubmed:34903130]
Nat Prod Res. 2021 Dec 14:1-4.
In this work was described the phytochemical investigation of Machaerium opacum Vogel (Fabaceae) leaves as well as the insecticidal activity of its crude extract and fractions against Atta sexdens Linnaeus (Hymenoptera: Formicidae). The phytochemical study led to the identification of alpha-amyrin, beta-amyrin, lupeol, phytol, isoMucronulatol and rutin, described for the first time in M. opacum and Mucronulatol. Insecticidal activity was assessed by the ingestion of the crude extract and fractions incorporated into an artificial diet at three different concentrations (0.2, 1.0 and 2.0 mg mL(-1)). Statistical analysis revealed that all the samples of M. opacum at all concentrations tested showed significant results when compared to the pure diet control.
Computational insights into the identification of a potent matrix metalloproteinase inhibitor from Indigofera aspalathoides to control cancer metastasis.[Pubmed:33927994]
3 Biotech. 2021 May;11(5):206.
Matrix metalloproteinases (MMPs) are the major proteolytic enzymes which assist in regulating the metastatic process by degrading the extracellular matrix proteins. In this study, we have investigated the anti-metastatic potential of major bioactive compounds in the medicinal plant Indigofera aspalathoides targeting matrix metalloproteinases (MMP2 & MMP9) and it's in silico pharmacokinetic profiles using computational studies. Indigofera aspalathoides (Sivanar vembu in Tamil) is a renowned medicinal herb in traditional Indian medicine which contains indigocarpan, Mucronulatol, indigocarpan diacetate, erythroxydiol X and erythroxydiol Y as the major constituents. The 3-dimensional structure of MMP2 and MMP9 was designed by using I-tasser and Modeller and it was validated by PROCHECK. The structures of Mucronulatol and indigocarpan have been retrieved from PubChem and indigocarpan diacetate, erythroxydiol X & Y were drawn by using Chemdraw Ultra 6.0. Batimastat was used as a positive control. Molecular docking was performed by using AutoDock 4.2 tools and AutoDock vina, an open-source program which signifies an effective interaction between the phytoligands and MMP2 & MMP9. From the results, AutoDock 4.2 have showed that indigocarpan possesses strong binding energy (DeltaG) of - 7.68 kcal/mol towards MMP2 and - 6.35 kcal/mol towards MMP9, whereas batimastat showed binding energy (DeltaG) of - 6.34 kcal/mol for MMP2 and - 5.66 kcal/mol for MMP9, meanwhile the results from AutoDock vina indicates that indigocarpan possesses strong binding energy (DeltaG) of - 8.0 kcal/mol towards MMP2 and - 8.2 kcal/mol towards MMP9, whereas batimastat showed binding energy (DeltaG) of - 7.2 kcal/mol for MMP2 and - 7.6 kcal/mol for MMP9. Also, the ADME and toxicity results suggest that the indigocarpan compound possesses a druggable pharmacokinetic potentiality and does not have carcinogenicity and Ames mutagenesis compared with other phytoligands. Hence, it is evident from our results that both AutoDock platforms strongly revealed that the phytoligand, indigocarpan possesses strong inhibitory activity against MMP2 and MMP9 to control cancer metastasis. Supplementary Information: The online version contains supplementary material available at 10.1007/s13205-021-02731-w.
Exploring natural compounds for the management of non-small cell lung cancer.[Pubmed:32722994]
Nat Prod Res. 2021 Dec;35(24):5879-5882.
A growing incidence of drug resistance and tumour proliferation in non-small cell lung cancer escalates the urge for potential lead molecules. The plant-derived natural compounds have played a pivotal role in potential therapeutic agents owing to its versatility and low toxicity over the past decades. In this study, we have executed an in-silico based screening of 1574 natural compounds against the beta-catenin via an integrated pharmacophore approach. Further investigation revealed that Mucronulatol and 7,4'-dihydroxyhomoisoflavanone possess a higher Glide score (-4.748 and -3.943 kcal/mol), binding affinity (-44.763 and -41.883 kcal/mol) alongside drug-likeness property than the iCRT5. Moreover, these compounds are reported to have cytotoxicity against lung cancer cell lines with an IC50 value of 6.74 microM and 8.99 microM respectively. Furthermore, dynamic studies were employed to determine the structural stability and we hope that the lead molecules proposed in this study could effectively inhibit the beta-catenin pathway associated with NSCLC.
In silico pharmacokinetic and molecular docking studies of small molecules derived from Indigofera aspalathoides Vahl targeting receptor tyrosine kinases.[Pubmed:25848167]
Bioinformation. 2015 Feb 28;11(2):73-84.
Angiogenesis is the formation of new blood vessels from preexisting vascular network that plays an important role in the tumor growth, invasion and metastasis. Anti-angiogenesis targeting tyrosine kinases such as vascular endothelial growth factor receptor 2 (VEGFR2) and platelet derived growth factor receptor beta (PDGFRbeta) constitutes a successful target for the treatment of cancer. In this work, molecular docking studies of three bioflavanoid such as indigocarpan, Mucronulatol, indigocarpan diacetate and two diterpenes namely erythroxydiol X and Y derived from Indigofera aspalathoides as PDGFRbeta and VEGFR2 inhibitors were performed using computational tools. The crystal structures of two target proteins were retrieved from PDB website. Among the five compounds investigated, indigocarpan exhibited potent binding energy DeltaG = -7.04 kcal/mol with VEGFR2 and DeltaG = -4.82 with PDGFRbeta compared to commercially available anti-angiogenic drug sorafenib (positive control). Our results strongly suggested that indigocarpan is a potent angiogenesis inhibitor as ascertained by its potential interaction with VEGFR2 and PDGFRbeta. This hypothesis provides a better insight to control metastasis by blocking angiogenesis.
Yeast alpha-glucosidase inhibition by isoflavones from plants of Leguminosae as an in vitro alternative to acarbose.[Pubmed:20734984]
J Agric Food Chem. 2010 Sep 22;58(18):9988-93.
In the course of searching for new classes of alpha-glucosidase inhibitors originated from natural resources, 11 kinds of isoflavones, i.e., medicarpin (1), formononetin (2), Mucronulatol (3), (3R)-calussequinone (5), (3R)-5'-methoxyvestitol (6), tectorigenin (7), biochanin A (8), tuberosin (9), calycosin (10), daidzein (11), and genistein (12), as well as a flavone, liquritigenin (4), were isolated as active principles responsible for the yeast alpha-glucosidase inhibitory activity from two leguminous plant extracts, i.e., the heartwood extract of Dalbergia odorifera and the roots extract of Pueraria thunbergiana. Each components (1-12) demonstrated a significantly potent inhibition on yeast alpha-glucosidase in a dose dependent manner when the p-nitrophenyl-alpha-D-glucopyranoside was used as a substrate in vitro. The concentration required for 50% enzyme inhibition (IC50) were calculated as 2.93 mM (1), 0.51 mM (2), 3.52 mM (7) 0.35 mM (8), 3.52 mM (9), 0.85 mM (11), and 0.15 mM (12) when that of reference drug acarbose was evaluated as 9.11 mM, in vitro. However, isoflavone glycosides, i.e., puerarin (13), daidzin (14), formononetin-7-O-beta-glucopyranoside (15), and genistin (16), exhibited a relatively poor inhibitory activity on yeast alpha-glucosidase as compared with the corresponding isoflavone (2, 11, 12), respectively.
Mucronulatol from Caribbean propolis exerts cytotoxic effects on human tumor cell lines.[Pubmed:18538108]
Int J Clin Pharmacol Ther. 2008 May;46(5):226-35.
OBJECTIVE: Mucronulatol is one of the most cytotoxic substances present in Caribbean propolis. This work aimed at initially characterizing the biological effects of Mucronulatol in cancer cell lines comprehending both wildtype and resistant sublines. MATERIALS AND METHODS: An RP-HPLC technique was employed to separate and purify Mucronulatol. IC(50) values were determined using the sulforhodamine B (SRB) proliferation assay. FACS-based cell cycle studies were carried out combining propidium iodide staining and 5-bromo-2'-deoxyuridine incorporation. Cell cycle regulator proteins were detected by Western blotting. The transcription of genes of interest was analyzed using RT-PCR. RESULTS: In MDR1-/MDR3+ cells, Mucronulatol exhibited cytotoxicity in the range of 2.7 - 10.2 microg/ml, while no cytotoxic effects were observed in MDR1+ systems at up to 100 microg/ml. Cytometric studies revealed that Mucronulatol promoted a global reduction in all cell cycle phases, with a remarkable increase of the apoptotic sub-G1 population. Immunoblotting showed that Mucronulatol induced an up-regulation of p21(Cip1) and p27(Kip1) while down-regulating cyclin E and CDK4 in a drug concentration-dependent manner. No effect on topoisomerase I was observed, while we detected an altered expression of topoisomerases II-I+/-/I(2). RT-PCR studies showed that 2-fold the IC(50) in HCT8 colon carcinoma cells was sufficient for altering the expression pattern of genes in this cell line, including topoisomerase I, thymidilate synthase, EGF receptor and c-myc, amongst others. CONCLUSION: Here, we demonstrate for the first time that Mucronulatol exerts cytotoxicity in cancer cell lines by targeting the control of cell cycle progression, indicating that the mechanism of action of this compound involves interference with the cell cycle machinery.
Cytotoxic constituents from Brazilian red propolis and their structure-activity relationship.[Pubmed:18440233]
Bioorg Med Chem. 2008 May 15;16(10):5434-40.
Several classes of flavonoids [flavanoids (1-10), flavonol (11), isoflavones (12-18), isoflavanones (19-22), isoflavans (23-26), chalcones (27-30), auronol (31), pterocarpans (32-37), 2-arylbenzofuran (38), and neoflavonoid (39)] and lignans (40-42) isolated from the MeOH extract of Brazilian red propolis were investigated for their cytotoxic activity against a panel of six different cancer cell lines including murine colon 26-L5 carcinoma, murine B16-BL6 melanoma, murine Lewis lung carcinoma, human lung A549 adenocarcinoma, human cervix HeLa adenocarcinoma, and human HT-1080 fibrosarcoma cell lines. Based on the observed results, structure-activity relationships were discussed. Among the tested compounds, 7-hydroxy-6-methoxyflavanone (3) exhibited the most potent activity against B16-BL6 (IC(50), 6.66microM), LLC (IC(50), 9.29microM), A549 (IC(50), 8.63microM), and HT-1080 (IC(50), 7.94microM) cancer cell lines, and Mucronulatol (26) against LLC (IC(50), 8.38microM) and A549 (IC(50), 9.9microM) cancer cell lines. These activity data were comparable to those of the clinically used anticancer drugs, 5-fluorouracil and doxorubicin, against the tested cell lines, suggesting that 3 and 26 are the good candidates for future anticancer drug development.
Bioactive flavonoids from the black locust tree, robinia pseudoacacia.[Pubmed:21214467]
Pharm Biol. 2000;38(3):229-34.
Five flavonoids, acacetin ( 1 ), secundiflorol I ( 2 ), Mucronulatol ( 3 ), isoMucronulatol ( 4 ), and isovestitol ( 5 ) were isolated, with the fractionation being guided by the brine shrimp lethality test (BST), from the ethanolic extract of the whole plant of Robinia pseudoacacia (Fabaceae). The structures of 1 - 5 were identified by spectral analyses. Compounds 2 - 5 are, for the first time, reported from this species. Corrections have been made for the previous literature assignments of the 13 C NMR resonances of compounds 1 - 3 . Bioactivities in BST and cytotoxicities against a panel of six solid human tumor cell lines were determined, and 1 was significantly cytotoxic in the prostate cell line (PC-3).
[Isolation and identification of chemical constituents of Astragalus root].[Pubmed:2092578]
Yao Xue Xue Bao. 1990;25(9):694-8.
Ten constituents have been isolated from the alcoholic extract of Astragalus membranaceus var. monghlicum root. All of them were identified. Among them astraisoflavanin (3S-(-)-Mucronulatol-7-O-D-glucopyranoside) is a new compound. Dimethyl 4,4'-dimethoxy-5,6,5',6'-dimethylenedioxybiphyenyl 1-2, 2'-dicarboxylate is a known synthetic compound, but it was first isolated from natural resource Astragalus root and identified by the authors.


