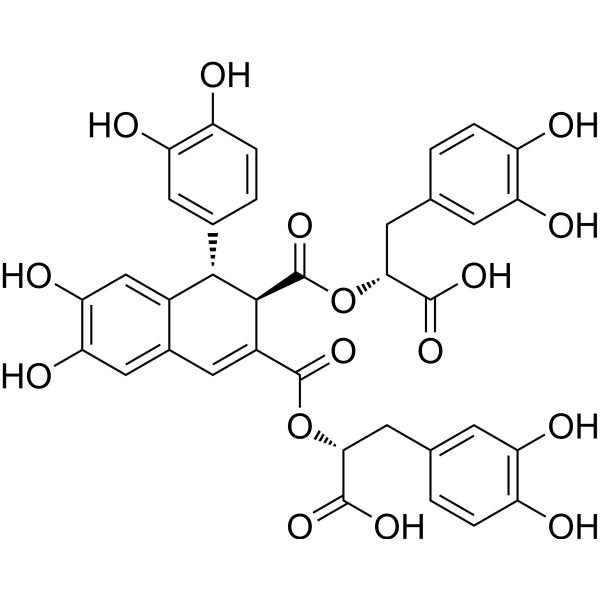
Caffeic acid tetramerCAS# 130286-75-8 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 130286-75-8 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C36H30O16 | M.Wt | 718.61 |
| Type of Compound | Other Phenolic Compounds | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Caffeic acid tetramer Dilution Calculator

Caffeic acid tetramer Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3916 mL | 6.9579 mL | 13.9158 mL | 27.8315 mL | 34.7894 mL |
| 5 mM | 0.2783 mL | 1.3916 mL | 2.7832 mL | 5.5663 mL | 6.9579 mL |
| 10 mM | 0.1392 mL | 0.6958 mL | 1.3916 mL | 2.7832 mL | 3.4789 mL |
| 50 mM | 0.0278 mL | 0.1392 mL | 0.2783 mL | 0.5566 mL | 0.6958 mL |
| 100 mM | 0.0139 mL | 0.0696 mL | 0.1392 mL | 0.2783 mL | 0.3479 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Celangulin V
Catalog No.:BCX1703
CAS No.:139979-81-0
- Alismanol M
Catalog No.:BCX1702
CAS No.:2408810-59-1
- Scutellarein-7-O-diglucuronide
Catalog No.:BCX1701
CAS No.:150641-65-9
- Homoveratric acid
Catalog No.:BCX1700
CAS No.:93-40-3
- Ladanetin-6-O-β-D-glucopyranoside
Catalog No.:BCX1699
CAS No.:89647-63-2
- Pedaliin 6''-acetate
Catalog No.:BCX1698
CAS No.:160789-41-3
- Cirsiliol 4'-glucoside
Catalog No.:BCX1697
CAS No.:41087-98-3
- Ladanetin-6-O-β-(6''-O- acetyl)glucoside
Catalog No.:BCX1696
CAS No.:1256959-27-9
- Complanatin II
Catalog No.:BCX1695
CAS No.:142449-94-3
- Apigenin-7-O-diglucuronoside
Catalog No.:BCX1694
CAS No.:119738-57-7
- Arborescosidic acid
Catalog No.:BCX1693
CAS No.:325798-51-4
- (+)-(3’S,4’R)-3’-acetyl-4’-isobutyrylkhellactone
Catalog No.:BCX1692
CAS No.:1562996-11-5
- (1α,2α,6β,8α,9α)-1,2,6,8,12-pentakis(acetyloxy)-9-(benzoyloxy)dihydro-β-agarofuran
Catalog No.:BCX1705
CAS No.:128443-60-7
- Epinodosinol
Catalog No.:BCX1706
CAS No.:27548-88-5
- Apigenin 4'-O-β-D-glucopyranoside
Catalog No.:BCX1707
CAS No.:20486-34-4
- Celangulatin C
Catalog No.:BCX1708
CAS No.:1000784-44-0
- Angulatin G
Catalog No.:BCX1709
CAS No.:1000784-46-2
- Angulatin E
Catalog No.:BCX1710
CAS No.:403613-20-7
- Protoapigenin 4'-O-β-D-glucoside
Catalog No.:BCX1711
CAS No.:163559-04-4
- 2-(trans-1,4-dihydroxy-cyclohexyl)-5,7-dihydroxy-chromone
Catalog No.:BCX1712
CAS No.:1270013-29-0
- Protoapigenin
Catalog No.:BCX1713
CAS No.:879325-07-2
- 2,3-Dihydro-3-hydroxy-2-oxo-1H-indole-3-acetic acid
Catalog No.:BCX1714
CAS No.:57061-17-3
- Wallichoside
Catalog No.:BCX1715
CAS No.:60657-36-5
- Rabdophyllin G
Catalog No.:BCX1716
CAS No.:82460-75-1
A new caffeic acid tetramer from the Dracocephalum moldavica L.[Pubmed:28805461]
Nat Prod Res. 2018 Feb;32(3):370-373.
A new Caffeic acid tetramer compound, named (+) methyl rabdosiin (4), together with seven known caffeic acid multimers (1-3, 5-8) and one caffeic acid monomer (9), were isolated from the aerial parts of Dracocephalum moldavica L. The structures of these compounds were assigned on the basis of 1D and 2D NMR spectroscopic and mass spectrometry analyses. The protective effects of compounds 2-4 against hydrogen peroxide (H(2)O(2))-induced apoptosis were evaluated in primary cardiomyocytes of SD neonatal rats in vitro by the MTT method. Three compounds exhibited potent protective activities at 12.5 mug/mL.
[Physicochemical stability and purification technology of caffeic acid tetramer from Arnebia euchroma].[Pubmed:18837312]
Zhongguo Zhong Yao Za Zhi. 2008 Jul;33(13):1552-5.
OBJECTIVE: To purify Caffeic acid tetramer (CAT) with macroporous resin on the basis of its fundamental physicochemical stability research. METHOD: The changes of CAT content were compared by HPLC method before and after the purification process, or while other conditions were altered. RESULT: LK001 was the best one among 7 kinds of macroporous resin in regard of purifying ability. The optimum absorbing technology was the solution concentration at 10 g x L(-1), pH at 4.5, and the flow rate at 3 BV x h(-1). The best eluting technology was 45% ethanol as eluting agent, pH at 5.0, eluting volume at 50 mL after applying super-purified water and 20% ethanol. The yield of product was 3. 6 percent, and the active compound CAT was 58 percent in the product. CONCLUSION: Macroporous resin LK001 is effective in enriching CAT from the crude extracts, thus this method of purification is advisable.
Biocatalytic properties of a peroxidase-active cell-free extract from onion solid wastes: caffeic acid oxidation.[Pubmed:18670892]
Biodegradation. 2009 Apr;20(2):143-53.
The exploitation of food residual sources consists of a major factor in reducing the polluting load of food industry wastes and developing novel added-value products. Plant food residues including trimmings and peels might contain a range of enzymes capable of transforming bio-organic molecules with potential phytotoxicity, including hydrolases, peroxidases and polyphenoloxidases. Although the use of bacterial and fungal enzymes has gained interest in studies pertaining to bioremediation applications, plant enzymes have been given less attention or even disregarded. In this view, this study aimed at the investigating the use of a crude peroxidase preparation from onion solid by-products for oxidising caffeic acid, a widespread o-diphenol, whose various derivatives may occur in food industry wastes, such as olive mill waste waters. Increased enzyme activity was observed at a pH value of 5, but considerable activity was also retained for pH up to 7. Favourable temperatures for increased activity varied between 20 degrees C and 40 degrees C, 30 degrees C being the optimal. Liquid chromatography-mass spectrometry analysis of a homogenate/H(2)O(2)-treated caffeic acid solution revealed the existence of a tetramer as major oxidation product. Based on the data generated, a putative pathway for the formation of the peroxidase-mediated Caffeic acid tetramer was proposed.


