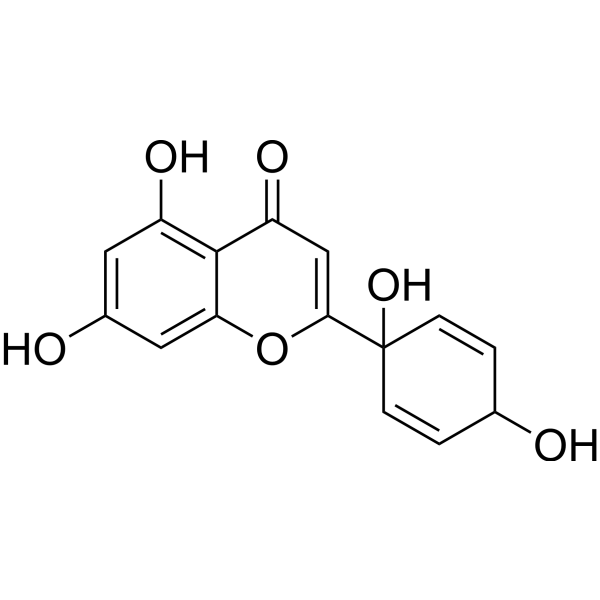
ProtoapigeninCAS# 879325-07-2 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 879325-07-2 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C15H12O6 | M.Wt | 288.25 |
| Type of Compound | Chromones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Protoapigenin Dilution Calculator

Protoapigenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4692 mL | 17.3461 mL | 34.6921 mL | 69.3842 mL | 86.7303 mL |
| 5 mM | 0.6938 mL | 3.4692 mL | 6.9384 mL | 13.8768 mL | 17.3461 mL |
| 10 mM | 0.3469 mL | 1.7346 mL | 3.4692 mL | 6.9384 mL | 8.673 mL |
| 50 mM | 0.0694 mL | 0.3469 mL | 0.6938 mL | 1.3877 mL | 1.7346 mL |
| 100 mM | 0.0347 mL | 0.1735 mL | 0.3469 mL | 0.6938 mL | 0.8673 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-(trans-1,4-dihydroxy-cyclohexyl)-5,7-dihydroxy-chromone
Catalog No.:BCX1712
CAS No.:1270013-29-0
- Protoapigenin 4'-O-β-D-glucoside
Catalog No.:BCX1711
CAS No.:163559-04-4
- Angulatin E
Catalog No.:BCX1710
CAS No.:403613-20-7
- Angulatin G
Catalog No.:BCX1709
CAS No.:1000784-46-2
- Celangulatin C
Catalog No.:BCX1708
CAS No.:1000784-44-0
- Apigenin 4'-O-β-D-glucopyranoside
Catalog No.:BCX1707
CAS No.:20486-34-4
- Epinodosinol
Catalog No.:BCX1706
CAS No.:27548-88-5
- (1α,2α,6β,8α,9α)-1,2,6,8,12-pentakis(acetyloxy)-9-(benzoyloxy)dihydro-β-agarofuran
Catalog No.:BCX1705
CAS No.:128443-60-7
- Caffeic acid tetramer
Catalog No.:BCX1704
CAS No.:130286-75-8
- Celangulin V
Catalog No.:BCX1703
CAS No.:139979-81-0
- Alismanol M
Catalog No.:BCX1702
CAS No.:2408810-59-1
- Scutellarein-7-O-diglucuronide
Catalog No.:BCX1701
CAS No.:150641-65-9
- 2,3-Dihydro-3-hydroxy-2-oxo-1H-indole-3-acetic acid
Catalog No.:BCX1714
CAS No.:57061-17-3
- Wallichoside
Catalog No.:BCX1715
CAS No.:60657-36-5
- Rabdophyllin G
Catalog No.:BCX1716
CAS No.:82460-75-1
- Angulatin K
Catalog No.:BCX1717
CAS No.:1631992-80-7
- (2E,4E)-8-Hydroxy-2,7-dimethyl-decadien-(2,4)-disaeure-(1,10)-dioic acid
Catalog No.:BCX1718
CAS No.:2808401-10-5
- Aristol-1(10)-en-9-ol
Catalog No.:BCX1719
CAS No.:114339-94-5
- Maritimetin
Catalog No.:BCX1720
CAS No.:576-02-3
- Polyporoid B
Catalog No.:BCX1721
CAS No.:1042362-45-7
- Polyporoid C
Catalog No.:BCX1722
CAS No.:1042362-47-9
- 2R-3',4',8-Trihydroxyflavanone-7-O-glucoside
Catalog No.:BCX1723
CAS No.:56389-87-8
- 2',3'-Dihydro-2'-hydroxyprotoapigenone
Catalog No.:BCX1724
CAS No.:1365655-88-4
- Angulatin B
Catalog No.:BCX1725
CAS No.:142546-07-4
[Flavonoids with special B-ring from Macrothelypteris viridifrons and their anti-proliferative effects on tumor cell].[Pubmed:21657076]
Zhongguo Zhong Yao Za Zhi. 2011 Mar;36(5):582-4.
OBJECTIVE: To study the chemical constituents of Macrothelypteris viridifrons and their anti-proliferative effects on tumor cell. METHOD: The compounds were isolated by column chromatography with silica gel, C18 reverse-phase silica gel, sephadex LH-20, and their structures were elucidated on the basis of physiochemical propertities and spectral analysis. The antitumor activities of all compounds were tested with MOLT4, Hep G2, A-549, MCF-7, HT-29, PC-3 tumor cell lines. RESULT: Five compounds were isolated and identified as protoapigenone (1), Protoapigenin (2), Protoapigenin-4'-O-beta-D-glucopyanoside (3), 5,7-dihydroxy-2-(1,2-isopropyldioxy-4-oxo-cyclohex-5-enyl) -chromen-4-one (4), 5,7-dihydroxy-2-(1-hydroxy-2,6-dimethoxy-cyclohex-4-oxo) -chromen-4-one (5), respectively. CONCLUSION: All compounds were obtained from this plant for the first time. Compounds 1, 4 and 5 showed strong anti-proliferative effects on six tumor cells, which were in concentration-dependent manner.
Flavonoids from the aerial parts of Macrothelypteris torresiana.[Pubmed:21240759]
Nat Prod Res. 2011 Jan;25(1):36-9.
Two new flavone derivatives (1 and 2) were isolated from the aerial parts of Macrothelypteris torresiana, along with four known flavonoids: Protoapigenin, apigenin, kaempferol and quercetin. The structures were determined on the basis of spectroscopic data. Compound 1 showed weak cytotoxic activity against human tumour cell lines HepG(2) , MCF(7) and K562.
Protoflavonoids from ferns impair centrosomal integrity of tumor cells.[Pubmed:20945277]
Planta Med. 2011 Mar;77(5):461-6.
Six protoflavonoids, including two new compounds, were isolated during a large scale screening of fern extracts for original interaction with mitosis. The new compounds isolated from PHEGOPTERIS decursive-pinnata and EQUISETUM fluviatile were 2',3'-dihydroprotogenkwanone (1) and 2',3'-dihydro-2'-hydroxyprotoapigenone (2). Known compounds were: protoapigenone, protogenkwanone, Protoapigenin, and 4'- O- beta-D-glucopyranosyl Protoapigenin. They showed a cytotoxic activity against HeLa cells at a micromolar level. IC(5)(0) values were 2 microM for compound 1 > 10 microM for compound 2, and respectively 2.4, 0.6, > 10 microM for the known compounds. Their cytotoxic effects were associated with phenotypic changes never observed before and characterized by the loss of centrosomal gamma-tubulin labelling in both mitotic and interphasic cells.
New cytotoxic flavonoids from Thelypteris torresiana.[Pubmed:16206043]
Planta Med. 2005 Sep;71(9):867-70.
During our search for anti-tumor agents from pteridophytes, three new flavonoids, protoapigenone (1), 5',6'-dihydro-6'-methoxyprotoapigenone (2), and Protoapigenin (3), along with four known compounds, Protoapigenin 4'- O-beta- D-glucoside (4), apigenin 4'- O-beta- D-glucoside (5), kaempferol 3- O-alpha- L-rhamnopyranoside (6), kaempferol 3,7-di- O-alpha- L-rhamnopyranoside (7), were isolated from Thelypteris torresiana using bioactivity-guided fractionation methods . The structures of the new isolates were elucidated by 1D- and 2D-NMR spectral analysis. Among the 7 compounds, protoapigenone (1) exhibited significant anti-tumor activities toward Hep G2, Hep 3B, MCF-7, A549, and MDA-MB-231 with IC50 values of 1.60, 0.23, 0.78, 3.88 and 0.27 microg/mL, respectively.


