MaritimetinCAS# 576-02-3 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 576-02-3 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
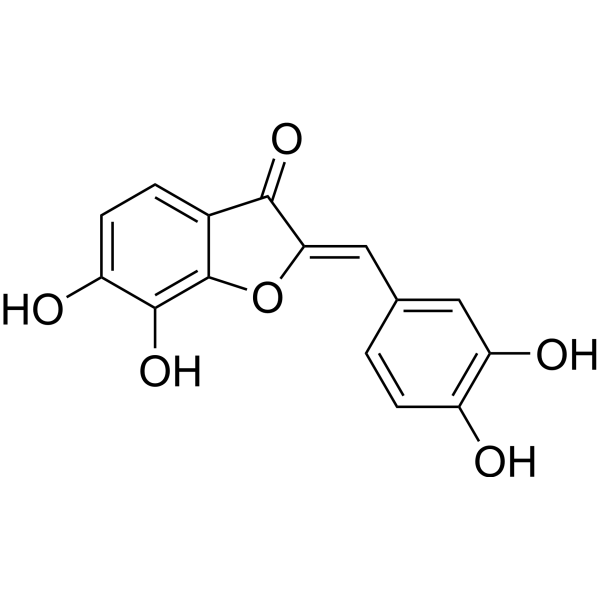
| Formula | C15H10O6 | M.Wt | 286.24 |
| Type of Compound | Aurones | Storage | Desiccate at -20°C |
| Synonyms | (Z)-6,7,3\',4\'-Tetrahydroxyaurone | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Maritimetin Dilution Calculator

Maritimetin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4936 mL | 17.4679 mL | 34.9357 mL | 69.8714 mL | 87.3393 mL |
| 5 mM | 0.6987 mL | 3.4936 mL | 6.9871 mL | 13.9743 mL | 17.4679 mL |
| 10 mM | 0.3494 mL | 1.7468 mL | 3.4936 mL | 6.9871 mL | 8.7339 mL |
| 50 mM | 0.0699 mL | 0.3494 mL | 0.6987 mL | 1.3974 mL | 1.7468 mL |
| 100 mM | 0.0349 mL | 0.1747 mL | 0.3494 mL | 0.6987 mL | 0.8734 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Aristol-1(10)-en-9-ol
Catalog No.:BCX1719
CAS No.:114339-94-5
- (2E,4E)-8-Hydroxy-2,7-dimethyl-decadien-(2,4)-disaeure-(1,10)-dioic acid
Catalog No.:BCX1718
CAS No.:2808401-10-5
- Angulatin K
Catalog No.:BCX1717
CAS No.:1631992-80-7
- Rabdophyllin G
Catalog No.:BCX1716
CAS No.:82460-75-1
- Wallichoside
Catalog No.:BCX1715
CAS No.:60657-36-5
- 2,3-Dihydro-3-hydroxy-2-oxo-1H-indole-3-acetic acid
Catalog No.:BCX1714
CAS No.:57061-17-3
- Protoapigenin
Catalog No.:BCX1713
CAS No.:879325-07-2
- 2-(trans-1,4-dihydroxy-cyclohexyl)-5,7-dihydroxy-chromone
Catalog No.:BCX1712
CAS No.:1270013-29-0
- Protoapigenin 4'-O-β-D-glucoside
Catalog No.:BCX1711
CAS No.:163559-04-4
- Angulatin E
Catalog No.:BCX1710
CAS No.:403613-20-7
- Angulatin G
Catalog No.:BCX1709
CAS No.:1000784-46-2
- Celangulatin C
Catalog No.:BCX1708
CAS No.:1000784-44-0
- Polyporoid B
Catalog No.:BCX1721
CAS No.:1042362-45-7
- Polyporoid C
Catalog No.:BCX1722
CAS No.:1042362-47-9
- 2R-3',4',8-Trihydroxyflavanone-7-O-glucoside
Catalog No.:BCX1723
CAS No.:56389-87-8
- 2',3'-Dihydro-2'-hydroxyprotoapigenone
Catalog No.:BCX1724
CAS No.:1365655-88-4
- Angulatin B
Catalog No.:BCX1725
CAS No.:142546-07-4
- Celangulin XIX
Catalog No.:BCX1726
CAS No.:403505-80-6
- Arabidopyl alcohol
Catalog No.:BCX1727
CAS No.:1400234-92-5
- 1α, 2α-diacetoxy-8β-isobutanoyloxy-9α-benzoyloxy-15-β-(β-furancarbonyloxy)-4β, 6β-dihydroxy-β-dihydr...
Catalog No.:BCX1728
CAS No.:2170562-63-5
- Phaseic acid
Catalog No.:BCX1729
CAS No.:24394-14-7
- (2E,4E)-2,4-Decadienoic acid
Catalog No.:BCX1730
CAS No.:30361-33-2
- Isochavicine
Catalog No.:BCX1731
CAS No.:30511-77-4
- Piperundecalidine
Catalog No.:BCX1732
CAS No.:88660-11-1
Snailase: A Promising Tool for the Enzymatic Hydrolysis of Flavonoid Glycosides From Plant Extracts.[Pubmed:35755698]
Front Plant Sci. 2022 Jun 9;13:889184.
Plants typically contain a broad spectrum of flavonoids in varying concentrations. As a rule, several flavonoid classes occur in parallel, and, even for a single flavonoid, divergent glycosylation patterns are frequently observed, many of which are not commercially available. This can be challenging in studies in which the distribution between flavonoid classes, or features that are not affected by glycosylation patterns, are adressed. In addition, hydrolysis simplifies the quantification process by reducing peak interferences and improving the peak intensity due to the accumulation of the respective aglycone. Effective removal of glycose moieties can also be relevant for technological applications of flavonoid aglycones. Herein, we present a fast and reliable method for the enzymatic hydrolysis glycosides from plant extracts using the commercial enzyme mix snailase, which provided the highest aglycone yields across all investigated flavonoids (aurones: leptosidin, Maritimetin, sulfuretin; chalcones: butein, lanceoletin, okanin, phloretin; dihydroflavonols: dihydrokaempferol; flavanones: eriodictyol, hesperetin; flavones: acacetin, apigenin, diosmetin, luteolin; flavonols: isorhamnetin, kaempferol, myricetin, quercetin; isoflavones: biochanin A, formononetin, genistein) from methanolic extracts of nine plants (Bidens ferulifolia, Coreopsis grandiflora, Fagus sylvatica, Malus x domestica, Mentha x piperita, Petunia x hybrida, Quercus robur, Robinia pseudoacacia, and Trifolium pratense) in comparison to four other enzymes (cellobiase, cellulase, beta-glucosidase, and pectinase), as well as to acidic hydrolysis by hydrochloric acid.
The chemical components of Coreopsis tinctoria Nutt. and their antioxidant, antidiabetic and antibacterial activities.[Pubmed:30499349]
Nat Prod Res. 2020 Jun;34(12):1772-1776.
Seventeen compounds were isolated from the capitula of Coreopsis tinctoria Nutt. with various column chromatographic methods and semi-preparative HPLC. Their structures were identified by the spectroscopic data and comparison with literatures as 2'-hydroxy-4,4'-dimethoxy-chalcone; (1), isoliquiritigenin (2), eriodictyol (3), naringenin (4), Maritimetin (5), butin (6), taxifolin (7), luteolin (8), 7,3',4'-trihydroxyflavone (9), 8,3',4'-trihydroxyflavone-7-O-beta-d-glucoside (10), quercetin (11), quercetagitin-7-O-beta-d-glucoside (12), quercetin-7-O-beta-d-glucoside (13), 3,4-dihydroxybenzoic acid (14), caffeic acid (15), coreoside B (16), and myo-inositol (17). Compounds 1, 4, 9, 10 and 17 were isolated from C. tinctoria Nutt. for the first time. Compounds 7 and 12 possessed the highest antioxidant activity (IC(50) = 64.37 and 32.86 microg/ml, respectively) among the tested compounds (IC(50) value of positive control was 5.34 microg/ml). Compound 7 exhibited potent PTP1B enzymatic inhibition with an IC(50) value of 7.73 mug/ml (IC(50) value of positive control is 1.46 microg/ml). Furthermore, compound 5 showed strong antibacterial activity against the Gram-positive bacterium, S. aureus.
New phenolic compounds from Coreopsis tinctoria Nutt. and their antioxidant and angiotensin i-converting enzyme inhibitory activities.[Pubmed:25516207]
J Agric Food Chem. 2015 Jan 14;63(1):200-7.
Three new phenolic compounds, coretinphenol (1), coretincone (2), and coretinphencone (3), were isolated from the buds of Coreopsis tinctoria Nutt., together with nine known compounds, including butein (4), okanin (5), isoliquiritigenin (6), Maritimetin (7), taxifolin (8), isookanin (9), marein (10), sachalinoside B (11), and 2-phenylethyl-beta-d-glucoside (12). The chemical structures of these compounds were elucidated by extensive spectroscopic analysis and on the basis of their chemical reactivity. This work represents the first recorded example of the isolation of compounds 1-3, 6, 7, 9, 11, and 12 from C. tinctoria. Compounds 5-9 showed strong diphenyl(2,4,6-trinitrophenyl)iminoazanium (DPPH) radical-scavenging activity, with IC50 values of 3.35 +/- 0.45, 9.6 +/- 2.32, 4.12 +/- 0.21, 6.2 +/- 0.43, and 7.9 +/- 0.53 muM, respectively. Compounds 2 and 8 exhibited angiotensin I-converting enzyme inhibitory activity, with IC50 values of 228 +/- 4.47 and 145.67 +/- 3.45 muM, respectively. The activities of phenolic compounds isolated from C. tinctoria support the medicinal use of this plant in the prevention of cardiovascular diseases.
[Chemical constituents from Bidens bipinnata].[Pubmed:25282892]
Zhongguo Zhong Yao Za Zhi. 2014 May;39(10):1838-44.
To investigate the chemical constituents of the whole plants of Bidens bipinnata, the separation and purification of constituents were performed by chromatography on macroporous resin, silica gel, MCI and Sephadex LH-20. Their structures were elucidated by spectroscopic data as quercetin (1), quercetin-3-0-alpha-L-rhamnoside (2), keampferol-3-O-beta-D-glucopyranoside (3), keampferol-3-O-alpha-L-rhamnoside (4), 3', 5-dyhydroxy-3, 6, 4'-trimethoxyl -7-O-beta-D-glucopyranoside flavonoid (5), 7, 8, 3', 4'-tetraflavanone(6), (2S)- and (2R)-isookanin-7-O-beta-D- glucopyranoside (7a/7b), (2S)- and (2R)-3'-methoxy-isookanin-8-O-beta-D-glucopyranoside (8a/8b), 6, 7, 3', 4'-tetrahydroxyaurone(9), Maritimetin (10), esculetin (11), 3-O-caffeoyl-2-methyl-d-erythrono-1, 4-lactone (12), (7S, 8R) balanophonin-4-O-beta-D-glucopyranoside (13), eugenyl-O-beta-apiofuranosyl-( 1"-6') -O-beta-glucopyranoside (14), and (+)-syringaresinol-4'-O-beta-D-glucopyranoside (15). Compounds 8, 13, 14, and 15 were isolated from this genus for the first time. Compounds 1 and 6 were potent inhibitors against HSC-T6 cells in vitro and compounds 1, 2, 6, and 7 were capable of decreasing the inflammatory cytokine production of macrophage cells in vitro.
4-Deoxyaurone formation in Bidens ferulifolia (Jacq.) DC.[Pubmed:23667445]
PLoS One. 2013 May 8;8(5):e61766.
The formation of 4-deoxyaurones, which serve as UV nectar guides in Bidens ferulifolia (Jacq.) DC., was established by combination of UV photography, mass spectrometry, and biochemical assays and the key step in aurone formation was studied. The yellow flowering ornamental plant accumulates deoxy type anthochlor pigments (6'-deoxychalcones and the corresponding 4-deoxyaurones) in the basal part of the flower surface whilst the apex contains only yellow carotenoids. For UV sensitive pollinating insects, this appears as a bicoloured floral pattern which can be visualized in situ by specific ammonia staining of the anthochlor pigments. The petal back side, in contrast, shows a faintly UV absorbing centre and UV absorbing rays along the otherwise UV reflecting petal apex. Matrix-free UV laser desorption/ionisation mass spectrometric imaging (LDI-MSI) indicated the presence of 9 anthochlors in the UV absorbing areas. The prevalent pigments were derivatives of okanin and Maritimetin. Enzyme preparations from flowers, leaves, stems and roots of B. ferulifolia and from plants, which do not accumulate aurones e.g. Arabidopsis thaliana, were able to convert chalcones to aurones. Thus, aurone formation could be catalyzed by a widespread enzyme and seems to depend mainly on a specific biochemical background, which favours the formation of aurones at the expense of flavonoids. In contrast to 4-hydroxyaurone formation, hydroxylation and oxidative cyclization to the 4-deoxyaurones does not occur in one single step but is catalyzed by two separate enzymes, chalcone 3-hydroxylase and aurone synthase (catechol oxidase reaction). Aurone formation shows an optimum at pH 7.5 or above, which is another striking contrast to 4-hydroxyaurone formation in Antirrhinum majus L. This is the first example of a plant catechol oxidase type enzyme being involved in the flavonoid pathway and in an anabolic reaction in general.
A DFT study on the radical scavenging activity of maritimetin and related aurones.[Pubmed:18983134]
J Phys Chem A. 2008 Nov 27;112(47):12196-202.
The radical scavenging activity of Maritimetin and a series of synthetic aurones has been studied by using density functional theory with the B3LYP exchange correlation functional. The computation of various molecular descriptors that could assist the elucidation of hydrogen atom and electron donating ability of the selected compounds was carried out in the gas phase and in the liquid phase (benzene, methanol, water) with the aid of IEF-PCM. For reasons of comparison a series of simple phenols of known activity were also included in the study. The results are discussed with regards to the structure-activity relationship principles of flavonoids and in particular to the capacity of the selected aurones to scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH(*)) and superoxide anion (O(2)(-*)) radicals. The O-H bond dissociation enthalpy (BDE) seems to be the most proper parameter to characterize the antiradical properties of the studied compounds. The hydroxylation pattern in ring B defines the order of activity, while the extended conjugation and especially the presence of a catechol moiety in ring A are responsible for the high activity observed experimentally for the selected aurones.


