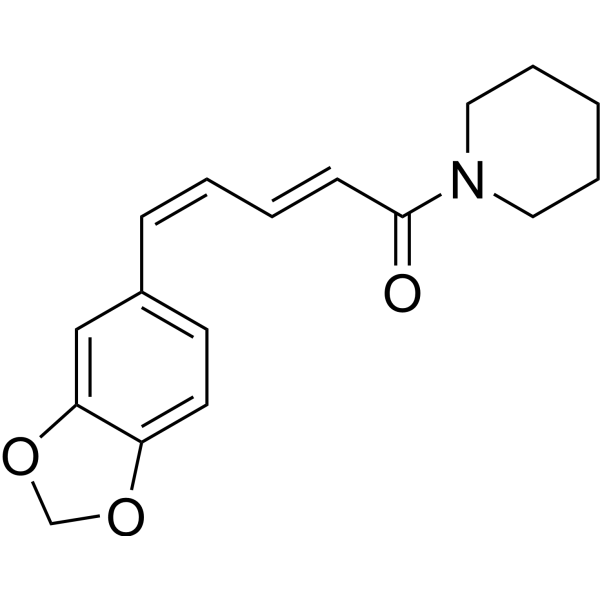
IsochavicineCAS# 30511-77-4 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 30511-77-4 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C17H19NO3 | M.Wt | 285.34 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isochavicine Dilution Calculator

Isochavicine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5046 mL | 17.523 mL | 35.0459 mL | 70.0918 mL | 87.6148 mL |
| 5 mM | 0.7009 mL | 3.5046 mL | 7.0092 mL | 14.0184 mL | 17.523 mL |
| 10 mM | 0.3505 mL | 1.7523 mL | 3.5046 mL | 7.0092 mL | 8.7615 mL |
| 50 mM | 0.0701 mL | 0.3505 mL | 0.7009 mL | 1.4018 mL | 1.7523 mL |
| 100 mM | 0.035 mL | 0.1752 mL | 0.3505 mL | 0.7009 mL | 0.8761 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (2E,4E)-2,4-Decadienoic acid
Catalog No.:BCX1730
CAS No.:30361-33-2
- Phaseic acid
Catalog No.:BCX1729
CAS No.:24394-14-7
- 1α, 2α-diacetoxy-8β-isobutanoyloxy-9α-benzoyloxy-15-β-(β-furancarbonyloxy)-4β, 6β-dihydroxy-β-dihydr...
Catalog No.:BCX1728
CAS No.:2170562-63-5
- Arabidopyl alcohol
Catalog No.:BCX1727
CAS No.:1400234-92-5
- Celangulin XIX
Catalog No.:BCX1726
CAS No.:403505-80-6
- Angulatin B
Catalog No.:BCX1725
CAS No.:142546-07-4
- 2',3'-Dihydro-2'-hydroxyprotoapigenone
Catalog No.:BCX1724
CAS No.:1365655-88-4
- 2R-3',4',8-Trihydroxyflavanone-7-O-glucoside
Catalog No.:BCX1723
CAS No.:56389-87-8
- Polyporoid C
Catalog No.:BCX1722
CAS No.:1042362-47-9
- Polyporoid B
Catalog No.:BCX1721
CAS No.:1042362-45-7
- Maritimetin
Catalog No.:BCX1720
CAS No.:576-02-3
- Aristol-1(10)-en-9-ol
Catalog No.:BCX1719
CAS No.:114339-94-5
- Piperundecalidine
Catalog No.:BCX1732
CAS No.:88660-11-1
- 15α-Hydroxy-20-oxo-6,7-seco-ent-kaur-16-en-1,7α(6,11α)-diolide
Catalog No.:BCX1733
CAS No.:1355450-84-8
- Celangulatin D
Catalog No.:BCX1734
CAS No.:1000784-45-1
- Retrofractamide A
Catalog No.:BCX1735
CAS No.:94079-67-1
- Ejaponine A
Catalog No.:BCX1736
CAS No.:1253119-28-6
- Celangulin
Catalog No.:BCX1737
CAS No.:116159-73-0
- Dihydrodehydrodiconiferyl alcohol 9-O-α-L-rhamnopyranoside
Catalog No.:BCX1738
CAS No.:1252572-36-3
- Dihydrodehydrodiconiferyl alcohol 9-O-β-D-xylopyranoside
Catalog No.:BCX1739
CAS No.:1015175-06-0
- 1α-Hydroxy-3-deoxypseudoanisatin
Catalog No.:BCX1740
CAS No.:258854-65-8
- Propionic acid (9R,10R)-9-acetoxy-8,8-dimethyl-9,10-dihydro-2H,8H-benzo[1,2-b:3,4- b′]dipyran-2-one-...
Catalog No.:BCX1741
CAS No.:440094-34-8
- (8R,8'R)-Matairesinol 4,4'-di-O-β-D-glucopyranoside
Catalog No.:BCX1742
CAS No.:41948-08-7
- Nortrachelogenin 4'-O-β-gentiobioside
Catalog No.:BCX1743
CAS No.:1961246-09-2
[Quantitative Analysis of Piperine and the Derivatives in Long Pepper Extract by HPLC Using Relative Molar Sensitivity].[Pubmed:31956239]
Shokuhin Eiseigaku Zasshi. 2019;60(5):134-143.
A novel method was developed for quantification of five major piperine derivatives (piperanine, piperine, chavicine, isopiperine, and Isochavicine) in a hot water extract of long pepper fruit (LPE) using the relative molar sensitivity (RMS) based on the combination of HPLC/UV and (1)H- quantitative NMR ((1)H-qNMR). The RMSs of piperanine, chavicine, isopiperine, and Isochavicine to piperine of which the absolute purity was determined by (1)H-qNMR were calculated to be 0.3693, 1.138, 0.9164, and 1.277, respectively. The total amount of piperine derivatives in LPE was quantified by both (1)H-qNMR and HPLC/UV based on the RMS using piperine as a single-reference material (RMS method). The relative difference in quantitation values of (1)H-qNMR and calibration curve method from the RMS method was 2.01% or less. The relative difference of the total cis-trans piperine isomers content between before and after photoirradiation in piperine solution was quantified to be 2.84% by the RMS method. In addition, the interlaboratory difference of the RMS method was confirmed in the range of 0.600 to 4.00 mug/g when analysis was performed on piperine derivatives in LPE containing tablets, while the total amount of piperine derivatives in the tablets was quantified at 606 mug/g. Our proposed method is a reliable tool for determining the contents of piperine and the derivatives in LPE and processed foods containing LPE.
Evaluation of drying process on the composition of black pepper ethanolic extract by high performance liquid chromatography with diode array detector.[Pubmed:24624176]
Jundishapur J Nat Pharm Prod. 2012 Fall;7(4):163-7. Epub 2012 Oct 7.
BACKGROUND: Black pepper (Piper nigrum) is one of the well-known spices extensively used worldwide especially in India, and Southeast Asia. The presence of alkaloids in the pepper, namely, piperine and its three stereoisomers, isopiperine, chavicine and Isochavicine are well noticed. OBJECTIVES: The current study evaluated the effect of lyophilization and oven drying on the stability and decomposition of constituents of black pepper ethanolic extract. MATERIALS AND METHODS: In the current study ethanolic extract of black pepper obtained by maceration method was dried using two methods. The effect of freeze and oven drying on the chemical composition of the extract especially piperine and its three isomers were evaluated by HPLC analysis of the ethanolic extract before and after drying processes using diode array detector. The UV Vis spectra of the peaks at piperine retention time before and after each drying methods indicated maximum absorbance at 341.2 nm corresponding to standard piperine. RESULTS: The results indicated a decrease in intensity of the chromatogram peaks at approximately all retention times after freeze drying, indicating a few percent loss of piperine and its isomers upon lyophilization. Two impurity peaks were completely removed from the extract. CONCLUSIONS: In oven dried samples two of the piperine stereoisomers were completely removed from the extract and the intensity of piperine peak was increased.
Leishmania donovani pteridine reductase 1: comparative protein modeling and protein-ligand interaction studies of the leishmanicidal constituents isolated from the fruits of Piper longum.[Pubmed:22752544]
J Mol Model. 2012 Dec;18(12):5065-73.
Visceral leishmaniasis or kala-azar is caused by the dimorphic parasite Leishmania donovani in the Indian subcontinent. Treatment options for kala-azar are currently inadequate due to various limitations. Currently, drug discovery for leishmaniases is oriented towards rational drug design; the aim is to identify specific inhibitors that target particular metabolic activities as a possible means of controlling the parasites without affecting the host. Leishmania salvages pteridin from its host and reduces it using pteridine reductase 1 (PTR1, EC 1.5.1.33), which makes this reductase an excellent drug target. Recently, we identified six alkamides and one benzenoid compound from the n-hexane fraction of the fruit of Piper longum that possess potent leishmanicidal activity against promastigotes as well as axenic amastigotes. Based on a homology model derived for recombinant pteridine reductase isolated from a clinical isolate of L. donovani, we carried out molecular modeling and docking studies with these compounds to evaluate their binding affinity. A fairly good agreement between experimental data and the results of molecular modeling investigation of the bioactive and inactive compounds was observed. The amide group in the conjugated alkamides and the 3,4-methylenedioxystyrene moiety in the benzenoid compound acts as heads and the long aliphatic chain acts as a tail, thus playing important roles in the binding of the inhibitor to the appropriate position at the active site. The remarkably high activity of a component containing piperine and piperine isomers (3.36:1) as observed by our group prompted us to study the activities of all four isomers of piperine-piperine (2E,4E), isopiperine (2Z,4E), Isochavicine (2E,4Z), and chavicine (2Z,4Z)-against LdPTR1. The maximum inhibitory effect was demonstrated by Isochavicine. The identification of these predicted inhibitors of LdPTR1 allowed us to build up a stereoview of the structure of the binding site in relation to activity, affording significant information that should prove useful during the structure-based design of leishmanicidal drugs.
[Phenolic and amide constituents from Lycianthes marlipoensis].[Pubmed:22256755]
Zhongguo Zhong Yao Za Zhi. 2011 Sep;36(18):2507-10.
Ten known phenolic compounds including [4]-gingerol (1), [6]-gingerol (2), [10]-gingerol (3), (3S,5S)-3,5-dihydroxy-1-(4-hydroxy-3-methoxyphenyl) decane (4), (3R,5S) -3, 5-dihydroxy-1-(4-hydroxy-3-methoxyphenyl) decane (5), [6]-shogaol (6), [10]-shogaol (7), gingerenone A (8), hexahydrocurcumin (9), and (3R,5R)-3,5-dihydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl) heptane (10), and seven amides including piperine (11), Isochavicine (12), isopiperine (13), N-trans-p-coumaroyl tyramine (14), N-trans-feruloyl tyramine (15), N-trans-p-coumaroyl octopamine (16), N-trans-feruloyl octopamine (17), were isolated and identified from the roots of Lycianthes marlipoensis. Compounds 1-13 and 17 were isolated from the genus Lycianthes for the first time.
Activation of TRPV1 and TRPA1 by black pepper components.[Pubmed:20460725]
Biosci Biotechnol Biochem. 2010;74(5):1068-72.
We searched in this study for novel agonists of transient receptor potential cation channel, subfamily V, member 1 (TRPV1) and transient receptor potential cation channel, subfamily A, member 1 (TRPA1) in pepper, focusing attention on 19 compounds contained in black pepper. Almost all the compounds in HEK cells heterogeneously expressed TRPV1 or TRPA1, increased the intracellular Ca(2+) concentration ([Ca(2+)](i)) in a concentration-dependent manner. Among these, piperine, isopiperine, Isochavicine, piperanine, pipernonaline, dehydropipernonaline, retrofractamide C, piperolein A, and piperolein B relatively strongly activated TRPV1. The EC(50) values of these compounds for TRPV1 were 0.6-128 microM. Piperine, isopiperine, Isochavicine, piperanine, piperolein A, piperolein B, and N-isobutyl-(2E,4E)-tetradeca-2,4-diamide also relatively strongly activated TRPA1, the EC(50) values of these compounds for TRPA1 were 7.8-148 microM. The Ca(2+) responses of these compounds for TRPV1 and TRPA1 were significantly suppressed by co-applying each antagonist. We identified in this study new transient receptor potential (TRP) agonists present in black pepper and found that piperine, isopiperine, Isochavicine, piperanine, piperolein A, and piperolein B activated both TRPV1 and TRPA1.
Kinetics of light-induced cis-trans isomerization of four piperines and their levels in ground black peppers as determined by HPLC and LC/MS.[Pubmed:17661483]
J Agric Food Chem. 2007 Aug 22;55(17):7131-9.
The pungent compounds piperine and isomers thereof, secondary metabolites present in black and white pepper fruit, undergo light-induced isomerizations. To facilitate studies in this area, an HPLC method has been developed for analysis and isolation of the following four possible piperine-derived photoinduced isomers: piperine, isopiperine, chavicine, and Isochavicine. The limits of detection (LOD) estimated from calibration plots were approximately 15-30 ng for each isomer. Reproducibilities of the analyses were excellent, and recoveries of spiked samples were as follows (average +/- SD; n = 3): chavicine, 98.4 +/- 2.1%; isopiperine, 96.2 +/- 3.2%; piperine, 104 +/- 3.8%; Isochavicine, 98.9 +/- 3.0%. To determine the kinetics of these isomerizations, fluorescent light, sunlight, and UV radiation at 254 nm was used to induce cis-trans geometric isomerization as a function of light intensities and time of exposure determined with the aid of high-performance liquid chromatography (HPLC) and liquid chromatography with diode array UV detection-mass spectrometry (LC-DAD/MS). HPLC was also used to determine the distribution of the isomers in four commercial ground black pepper products used as spices in culinary practice. Isomerization increased with light intensities and time of exposure and leveled off at the so-called photostationary phases. The piperine levels of the four products were quite similar, ranging (in wt %) from 10.17 to 11.68. The amounts of the other three isomers ranged from 0.01 to 0.07 of the total for chavicine; from 0.15 to 0.23 for isopiperine; and from 0.37 to 0.42 for Isochavicine. The results establish the utility of the HPLC method for simultaneous analysis of the four isomers both in pure form and in black pepper extracts. The dietary significance of the results is discussed.


