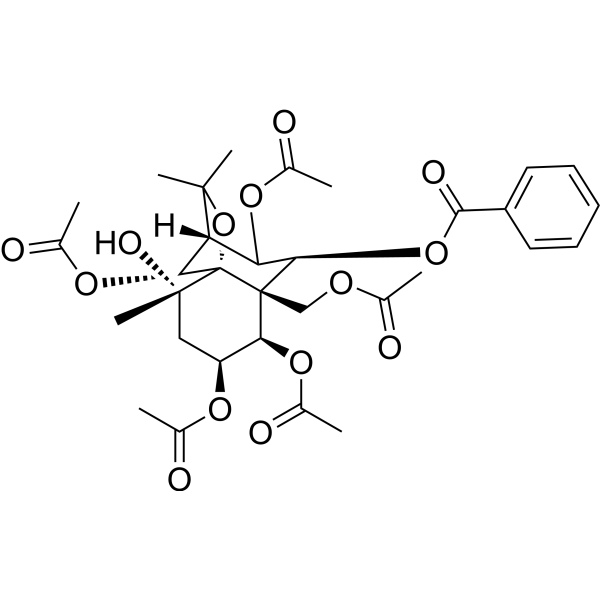
CelangulinCAS# 116159-73-0 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 116159-73-0 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C32H40O14 | M.Wt | 648.65 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Celangulatin,Angulatin J | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Celangulin Dilution Calculator

Celangulin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5417 mL | 7.7083 mL | 15.4166 mL | 30.8333 mL | 38.5416 mL |
| 5 mM | 0.3083 mL | 1.5417 mL | 3.0833 mL | 6.1667 mL | 7.7083 mL |
| 10 mM | 0.1542 mL | 0.7708 mL | 1.5417 mL | 3.0833 mL | 3.8542 mL |
| 50 mM | 0.0308 mL | 0.1542 mL | 0.3083 mL | 0.6167 mL | 0.7708 mL |
| 100 mM | 0.0154 mL | 0.0771 mL | 0.1542 mL | 0.3083 mL | 0.3854 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ejaponine A
Catalog No.:BCX1736
CAS No.:1253119-28-6
- Retrofractamide A
Catalog No.:BCX1735
CAS No.:94079-67-1
- Celangulatin D
Catalog No.:BCX1734
CAS No.:1000784-45-1
- 15α-Hydroxy-20-oxo-6,7-seco-ent-kaur-16-en-1,7α(6,11α)-diolide
Catalog No.:BCX1733
CAS No.:1355450-84-8
- Piperundecalidine
Catalog No.:BCX1732
CAS No.:88660-11-1
- Isochavicine
Catalog No.:BCX1731
CAS No.:30511-77-4
- (2E,4E)-2,4-Decadienoic acid
Catalog No.:BCX1730
CAS No.:30361-33-2
- Phaseic acid
Catalog No.:BCX1729
CAS No.:24394-14-7
- 1α, 2α-diacetoxy-8β-isobutanoyloxy-9α-benzoyloxy-15-β-(β-furancarbonyloxy)-4β, 6β-dihydroxy-β-dihydr...
Catalog No.:BCX1728
CAS No.:2170562-63-5
- Arabidopyl alcohol
Catalog No.:BCX1727
CAS No.:1400234-92-5
- Celangulin XIX
Catalog No.:BCX1726
CAS No.:403505-80-6
- Angulatin B
Catalog No.:BCX1725
CAS No.:142546-07-4
- Dihydrodehydrodiconiferyl alcohol 9-O-α-L-rhamnopyranoside
Catalog No.:BCX1738
CAS No.:1252572-36-3
- Dihydrodehydrodiconiferyl alcohol 9-O-β-D-xylopyranoside
Catalog No.:BCX1739
CAS No.:1015175-06-0
- 1α-Hydroxy-3-deoxypseudoanisatin
Catalog No.:BCX1740
CAS No.:258854-65-8
- Propionic acid (9R,10R)-9-acetoxy-8,8-dimethyl-9,10-dihydro-2H,8H-benzo[1,2-b:3,4- b′]dipyran-2-one-...
Catalog No.:BCX1741
CAS No.:440094-34-8
- (8R,8'R)-Matairesinol 4,4'-di-O-β-D-glucopyranoside
Catalog No.:BCX1742
CAS No.:41948-08-7
- Nortrachelogenin 4'-O-β-gentiobioside
Catalog No.:BCX1743
CAS No.:1961246-09-2
- Ophiopogonin R
Catalog No.:BCX1744
CAS No.:1418183-25-1
- Qianhucoumarin D
Catalog No.:BCX1745
CAS No.:20516-19-2
- Peucedanol
Catalog No.:BCX1746
CAS No.:46992-81-8
- Oxyresveratrol 4'-O-β-D-glucopyranoside
Catalog No.:BCX1747
CAS No.:863329-68-4
- Tanegoside A
Catalog No.:BCX1748
CAS No.:131653-21-9
- Chavicol 1-O-β-D-glucopyranoside
Catalog No.:BCX1749
CAS No.:64703-98-6
Synthesis, and Insecticidal Activities of Propargyloxy-Diphenyl Oxide-Sulfonamide Derivatives.[Pubmed:38380820]
Chem Biodivers. 2024 Apr;21(4):e202400206.
Agricultural pests are the primary contributing factor to crop yield reduction, particularly in underdeveloped regions. Despite the significant efficacy of pesticides in pest control, their extensive use has led to the drug-fast of insecticide resistance. Developing of new environmentally friendly plant-based pesticides is an urgent necessity. In this study, a series of diaryl ether compounds containing propargyloxy and sulfonamide groups were designed. The synthesis of these 36 compounds primarily relied on nuclear magnetic resonance for structure determination, while single-crystal X-ray diffraction was employed for certain compounds. Meanwhile, the insecticidal activities against Mythimna separata were also assessed. Some of the compounds exhibited significantly enhanced activity, the LC(50) value of the highest activity compound TD(8) (0.231 mg/mL) demonstrating respective increases by 100-fold compared to the plant pesticide Celangulin V (23.9 mg/mL), and a 5-fold increase with the positive control L-1 (1.261 mg/mL). The interaction between the target compound and the target, as well as the consistency of the target, were verified through symptomological analysis and molecular docking. The structure-activity relationships were also conducted. This study offered a novel trajectory for the advancement and formulation of future pesticides.
Diversifying the benzenesulfonamide scaffold for potential V-ATPase inhibitors: synthesis and insecticidal activity evaluation.[Pubmed:38319483]
Mol Divers. 2024 Feb 6.
Celangulin V is a natural beta-dihydroagarofuran derivative isolated from Celastrus angulatus which shows insecticidal activity in many agricultural pests. Using Celangulin V as a molecular probe, we find out a new pesticide target: subunit H of V-ATPase. To explore the potential application of this novel target, lead sulfonamides have been found through virtual screening. Combined with the previous work, 46 sulfonamide derivatives are designed and synthesized. All target compounds are first screened for their insecticidal activities against Mythimna separata. The results of bioassay reveal that most of the designed compounds exhibit significant insecticidal activities against third-instar larvae of M. separata under the concentration of 10 mg/mL, and compound 8.4 shows the highest activity with LC(50) value of 1.72 mg/mL, 15-fold smaller than that of Celangulin V (25.89 mg/mL). Molecular docking results further indicated that compound 8.4 might serve as a potential inhibitor of the subunit H of V-ATPase. This study provides a potential sulfonamide candidate compound for the M. separata control.
The Effect of Botanical Pesticides Azadirachtin, Celangulin, and Veratramine Exposure on an Invertebrate Species Solenopsis invicta (Hymenoptera: Formicidae).[Pubmed:38276530]
Toxins (Basel). 2023 Dec 20;16(1):6.
The injudicious and excessive use of synthetic pesticides has deleterious effects on humans, ecosystems, and biodiversity. As an alternative to traditional crop-protection methods, botanical pesticides are gaining importance. In this research endeavor, we examined the contact toxicity, knockdown time, lethal time, and toxicity horizontal transmission of three natural pesticides from plants (azadirachtin, Celangulin, and veratramine) on red imported fire ants (RIFA; Solenopsis invicta). Our research findings indicated that azadirachtin and Celangulin exhibited relatively high toxicity, with median lethal dose (LD(50)) values of 0.200 and 0.046 ng/ant, respectively, whereas veratramine exhibited an LD(50) value of 544.610 ng/ant for large workers of S. invicta at 24 h post-treatment. Upon treatment with 0.125 mg/L, the (median lethal time) LT(50) values of azadirachtin and Celangulin were determined to be 60.410 and 9.905 h, respectively. For veratramine, an LT(50) value of 46.967 h was achieved after being tested with 200 mg/L. Remarkably, azadirachtin and Celangulin were found to exhibit high horizontal transfer among RIFA, with high secondary mortality (100%) and tertiary mortalities (>61%) after 48 h of treatment with 250 mg/L, as well as with their dust formulations for 72 h. However, veratramine did not exhibit significant toxicity or horizontal transfer effects on RIFA, even at high concentrations. These findings suggest that azadirachtin and Celangulin are likely to have a highly prominent potential in the management of S. invicta.
Design, Synthesis, Insecticidal Activities and Molecular Docking of Sulfonamide Derivatives Containing Propargyloxy or Pyridine Groups.[Pubmed:36536172]
Chem Biodivers. 2023 Feb;20(2):e202201020.
The discovery of new highly active molecules from natural products is a common method to create new pesticides. Celangulin V targeting Mythimna separate (M. separate) midgut V-ATPase H subunit, has received considerable attention for its excellent insecticidal activity and unique mechanism of action. Therefore, combined with our preliminary work, thirty-seven sulfonamide derivatives bearing propargyloxy or pyridine groups were systematically synthesized to search for insecticidal candidate compounds with low cost and high efficiency on the H subunit of V-ATPase. Bioactive results showed that compounds A2-A4 and A6-A7 exhibited a better bioactivity with median effective concentration (LC(50) ) values (2.78, 3.11, 3.34, 3.54 and 2.48 mg/mL, respectively) against third-instar larvae of M. separate than Celangulin V (LC(50) =18.1 mg/mL). Additionally, molecular docking experiments indicated that these molecules may act on the H subunit of V-ATPase. Based on the above results, these compounds provide new ideas for the discovery of insecticides.
Design, synthesis and insecticidal activity of benzenesulfonamide derivatives containing various alkynyl, alkenyl and cyclopropyl groups in para position.[Pubmed:36200705]
Nat Prod Res. 2024 Jan-Feb;38(3):549-553.
Celangulin V is a natural beta-dihydrofuran sesquiterpene polyester with anti Mythimna separate activity and unique mechanism of action. Further study showed that its target was the H subunit of V-ATPase in the midgut of M. separate. Thus, combined with the previous work, thirty-two benzene sulfonamide derivatives were systematically synthesised to discover efficient and low-budget insecticidal candidates for the H subunit of V-ATPase. Screening results showed that compounds C2, C4, C5, C6 and C8 could significantly cause death of tested third-instar larvae of M. separate, and provided the corresponding LC50 values of 0.844, 0.953, 0.705, 0.599 and 0.887 mg/mL, which were extremely better than Celangulin V (LC50 = 11.5 mg/mL). The docking results indicated that this novel framework might target H subunit of V-ATPase. Given these excellent bioactivity results, this kind of sulfonamide framework could provide a suitable point for exploring highly efficient insecticidal agents.
Design, synthesis and insecticidal activities of 4-propargyloxybenzene sulfonamide derivatives substituted with amino acids.[Pubmed:35866233]
J Asian Nat Prod Res. 2023 Apr;25(4):379-386.
Sixty-nine 4-propargyloxybenzene sulfonamide derivatives with different amino acids as amino substituent were synthesized and evaluated for their insecticidal activity against third-instar Mythimna separate. The bioassay results revealed that some derivatives bearing amino acid ester group performed good insecticidal activity against third-instar M.separata, such as the LC(50) values of D18 and D19 were 4.28 and 2.96 mg/ml after 48 h, in particular, the LC(50) of D16 was 2.38 mg/ml and the activity was improved by 14 times compared to Celangulin V (34.48 mg/ml). The above results provided theoretical and experimental basis for the discovery of novel insecticidal active compounds.
Research Progress on the Synthetic Biology of Botanical Biopesticides.[Pubmed:35621485]
Bioengineering (Basel). 2022 May 12;9(5):207.
The production and large-scale application of traditional chemical pesticides will bring environmental pollution and food safety problems. With the advantages of high safety and environmental friendliness, botanical biopesticides are in line with the development trend of modern agriculture and have gradually become the mainstream of modern pesticide development. However, the traditional production of botanical biopesticides has long been faced with prominent problems, such as limited source and supply, complicated production processes, and excessive consumption of resources. In recent years, the rapid development of synthetic biology will break through these bottlenecks, and many botanical biopesticides are produced using synthetic biology, such as emodin, Celangulin, etc. This paper reviews the latest progress and application prospect of synthetic biology in the development of botanical pesticides so as to provide new ideas for the analysis of synthetic pathways and heterologous and efficient production of botanical biopesticides and accelerate the research process of synthetic biology of natural products.
Isolation and structural identification of insecticidal compounds from Tripterygium wilfordii.[Pubmed:34251917]
J Asian Nat Prod Res. 2022 Jul;24(7):648-656.
Five compounds were identified from Tripterygium wilfordii, including two novel compounds and three previously known compounds. Two newly discovered compounds are Celangulin CY (1alpha,2alpha,3beta,4beta,6beta,8alpha,13-hepacetoxy-9beta-benzoyloxy-beta-dihydroagarofuran) and Celangulin CQ (1alpha-nicotinoyloxy-2alpha,3beta,6beta-triacetoxy-9beta-furancarbonyloxy-13-isobutanoyloxy-4beta-hydroxy-beta-dihydroagarofuran). Their structures were determined using nuclear magnetic resonance (NMR), mass spectrometry (MS), and high-pressure liquid chromatography (HPLC). The isolated compounds were tested for insecticidal activity against the third instar larvae of Spodoptera frugiperda. Both Celangulin CY and Celangulin CQ exhibited significantly higher oral toxicity in the larvae than that exhibited by the three known compounds.
Design, synthesis, and insecticidal activities of propargyloxy-naphthalene-sulfonamide derivatives.[Pubmed:34042537]
J Asian Nat Prod Res. 2022 Apr;24(4):361-370.
In our previous studies, a kind of novel benzenesulfonamides was found to be a candidate insecticidal compounds. It was shown that propargyloxy and sulfonamide groups are pharmacodynamic groups. One hundred and twenty-six (126) naphthalenesulfonamides derivatives with propargyloxy functionality were designed and synthesized, and their insecticidal activities were determined. Some of them showed outstanding activity, with LC(50) values as low as 0.202 mg ml(-1), much lower than that of the positive control Celangulin V (23.9 mg ml(-1)). In addition, the structure-activity relationships were discussed, and molecular docking was used to verify the binding mode of the compound and the target receptor.
The Inhibitory Effect of Celangulin V on the ATP Hydrolytic Activity of the Complex of V-ATPase Subunits A and B in the Midgut of Mythimna separata.[Pubmed:30813232]
Toxins (Basel). 2019 Feb 22;11(2):130.
Celangulin V (CV) is a compound isolated from Celastrus angulatus Max that has a toxic activity against agricultural insect pests. CV can bind to subunits a, H, and B of the vacuolar ATPase (V-ATPase) in the midgut epithelial cells of insects. However, the mechanism of action of CV is still unclear. In this study, the soluble complex of the V-ATPase A subunit mutant TSCA which avoids the feedback inhibition by the hydrolysate ADP and V-ATPase B subunit were obtained and then purified using affinity chromatography. The H(+)K(+)-ATPase activity of the complex and the inhibitory activity of CV on ATP hydrolysis were determined. The results suggest that CV inhibits the ATP hydrolysis, resulting in an insecticidal effect. Additionally, the homology modeling of the AB complex and molecular docking results indicate that CV can competitively bind to the AB complex at the ATP binding site, which inhibits ATP hydrolysis. These findings suggest that the AB subunits complex is one of the potential targets for CV and is important for understanding the mechanism of interaction between CV and V-ATPase.
De novo leaf and root transcriptome analysis to explore biosynthetic pathway of Celangulin V in Celastrus angulatus maxim.[Pubmed:30611193]
BMC Genomics. 2019 Jan 5;20(1):7.
BACKGROUND: Celastrus angulatus Maxim is a kind of crucial and traditional insecticidal plant widely distributed in the mountains of southwest China. Celangulin V is the efficient insecticidal sesquiterpenoid of C. angulatus and widely used in pest control in China, but the low yield and discontinuous supply impeded its further popularization and application. Fortunately, the development of synthetic biology provided an opportunity for sustainable supply of Celangulin V, for which understanding its biosynthetic pathway is indispensable. RESULTS: In this study, six cDNA libraries were prepared from leaf and root of C. angulatus before global transcriptome analyses using the BGISEQ-500 platform. A total of 104,950 unigenes were finally obtained with an average length of 1200 bp in six transcriptome databases of C. angulatus, in which 51,817 unigenes classified into 25 KOG classifications, 39,866 unigenes categorized into 55 GO functional groups, and 48,810 unigenes assigned to 135 KEGG pathways, 145 of which were putative biosynthetic genes of sesquiterpenoid and triterpenoid. 16 unigenes were speculated to be related to Celangulin V biosynthesis. De novo assembled sequences were verified by Quantitative Real-Time PCR (qRT-PCR) analysis. CONCLUSIONS: This study is the first report on transcriptome analysis of C. angulatus, and 16 unigenes probably involved in the biosynthesis of Celangulin V were finally collected. The transcriptome data will make great contributions to research for this specific insecticidal plant and the further gene mining for biosynthesis of Celangulin V and other sesquiterpene polyol esters.
Synthesis and insecticidal activity in vitro and vivo of novel benzenesulfonyl derivatives based on potent target subunit H of V-ATPase.[Pubmed:30172616]
Bioorg Med Chem Lett. 2018 Oct 15;28(19):3164-3167.
Two lead compounds with benzenesulfonamide were found through virtual screening based on the 3D structure of the subunit H of V-ATPase in previous study. 74 benzenesulfonyl derivatives were synthesized and their insecticidal activities were evaluated. The derivatives with propargyl substituents exhibit excellent insecticidal activities against Mythimna separata Walker. The LD(50) values of compounds A5.7 (28.0 mug.g(-1)) and B5.7 (36.4 mug.g(-1)) were significantly less than that of Celangulin V (344.0 mug.g(-1)). Furthermore, Isothermal Titration Calorimetry (ITC) data indicate there is a strong binding affinity between A5.7 and V-ATPase Subunit H. These results demonstrate that it is a practical way to develop pesticides targeting at H subunit of V-ATPase.
Insight into the Mode of Action of Celangulin V on the Transmembrane Potential of Midgut Cells in Lepidopteran Larvae.[Pubmed:29210984]
Toxins (Basel). 2017 Dec 6;9(12):393.
Celangulin V (CV) is the main insecticidal constituent of Celastrus angulatus. The V-ATPase H subunit of the midgut cells of lepidopteran larvae is the putative target protein of CV. Here, we compared the effects of CV on the midgut membrane potentials of Mythimna separata and Agrotis ipsilon larvae with those of the Cry1Ab toxin from Bacillus thuringiensis and with those of inactive CV-MIA, a synthetic derivative of CV. We investigated the changes in the apical membrane potentials (V(am)) and basolateral membrane potentials (V(bm)) of the midguts of sixth-instar larvae force-fed with the test toxins. We also measured the V(am) and V(bm) of larval midguts that were directly incubated with the test toxins. Similar to the effect of Cry1Ab, the V(am) of CV-treated midguts rapidly decayed over time in a dose-dependent manner. By contrast, CV-MIA did not influence V(am). Meanwhile, the V(am) of A. ipsilon larval midguts directly incubated with CV decayed less than that of M. separata larval midguts, whereas that of larvae force-fed with CV did not significantly change. Similar to Cry1Ab, CV did not affect the V(bm) of isolated midguts. CV significantly inhibited V-ATPase activity in a dose-dependent manner. Therefore, CV initially inhibits V-ATPase in the apical membrane and affects intracellular pH, homeostasis, and nutrient transport mechanisms in lepidopteran midgut cells.
Molecular Insights into the Potential Insecticidal Interaction of beta-Dihydroagarofuran Derivatives with the H Subunit of V-ATPase.[Pubmed:29019960]
Molecules. 2017 Oct 11;22(10):1701.
Celangulin V (CV), one of dihydroagarofuran sesquiterpene polyesters isolated from Chinese bittersweet (Celastrus angulatus Maxim), is famous natural botanical insecticide. Decades of research suggests that is displays excellent insecticidal activity against some insects, such as Mythimna separata Walker. Recently, it has been validated that the H subunit of V-ATPase is one of the target proteins of the insecticidal dihydroagarofuran sesquiterpene polyesters. As a continuation of the development of new pesticides from these natural products, a series of beta-dihydroagarofuran derivatives have been designed and synthesized. The compound JW-3, an insecticidal derivative of CV with a p-fluorobenzyl group, exhibits higher insecticidal activity than CV. In this study, the potential inhibitory effect aused by the interaction of JW-3 with the H subunit of V-ATPase c was verified by confirmatory experiments at the molecular level. Both spectroscopic techniques and isothermal titration calorimetry measurements showed the binding of JW-3 to the subunit H of V-ATPase was specific and spontaneous. In addition, the possible mechanism of action of the compound was discussed. Docking results indicated compound JW-3 could bind well in 'the interdomain cleft' of the V-ATPase subunit H by the hydrogen bonding and make conformation of the ligand-protein complex become more stable. All results are the further validations of the hypothesis, that the target protein of insecticidal dihydroagarofuran sesquiterpene polyesters and their beta-dihydroagarofuran derivatives is the subunit H of V-ATPase. The results also provide new ideas for developing pesticides acting on V-ATPase of insects.
Insecticidal Pregnane Glycosides from the Root Barks of Periploca sepium.[Pubmed:30549591]
Nat Prod Commun. 2016 Oct;11(10):1425-1428.
To explore novel lead compounds for botanical pesticides from natural sources, a new pregnane glycoside (periplocoside) P2 as well as its isomer, a known pregnane glycoside P1, were isolated from the root barks of Periploca sepium using a bioactivity-guided method, and their structures were confirmed by ID NMR, 2D NMR, IR, ESI-MSn and HRMS. Their insecticidal activities were evaluated against 3rd instar larvae of M separata, and the results indicated that P2 exhibited excellent insecticidal activity with LC(5)(0) values of 2.9 and 2.2 mg/mL after 24 and 48 h, respectively, much lower than those of the positive control Celangulin-V (24.7 and 21.0 mg/mL after 24 and 48 h, respectively). This work demonstrated that pregnane glycosides from Periploca sepium could be promising lead compounds for developing botanical pesticides urgently needed in agriculture.


