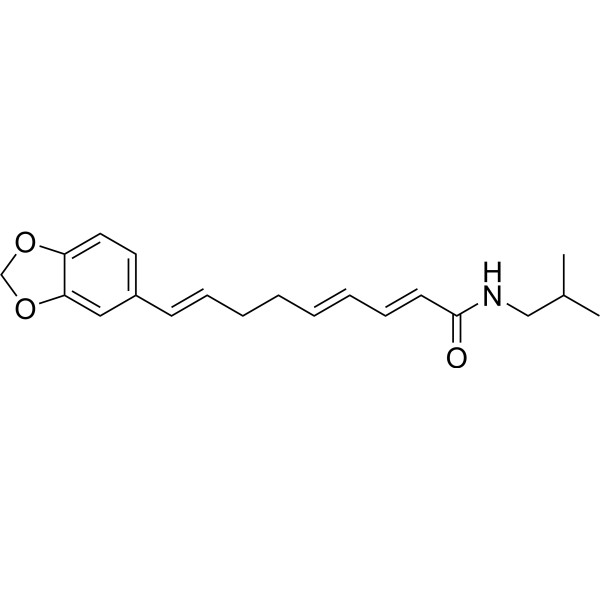
Retrofractamide ACAS# 94079-67-1 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 94079-67-1 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C20H25NO3 | M.Wt | 327.42 |
| Type of Compound | Amides | Storage | Desiccate at -20°C |
| Synonyms | Piperlongumine A | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Retrofractamide A Dilution Calculator

Retrofractamide A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0542 mL | 15.2709 mL | 30.5418 mL | 61.0836 mL | 76.3545 mL |
| 5 mM | 0.6108 mL | 3.0542 mL | 6.1084 mL | 12.2167 mL | 15.2709 mL |
| 10 mM | 0.3054 mL | 1.5271 mL | 3.0542 mL | 6.1084 mL | 7.6355 mL |
| 50 mM | 0.0611 mL | 0.3054 mL | 0.6108 mL | 1.2217 mL | 1.5271 mL |
| 100 mM | 0.0305 mL | 0.1527 mL | 0.3054 mL | 0.6108 mL | 0.7635 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Celangulatin D
Catalog No.:BCX1734
CAS No.:1000784-45-1
- 15α-Hydroxy-20-oxo-6,7-seco-ent-kaur-16-en-1,7α(6,11α)-diolide
Catalog No.:BCX1733
CAS No.:1355450-84-8
- Piperundecalidine
Catalog No.:BCX1732
CAS No.:88660-11-1
- Isochavicine
Catalog No.:BCX1731
CAS No.:30511-77-4
- (2E,4E)-2,4-Decadienoic acid
Catalog No.:BCX1730
CAS No.:30361-33-2
- Phaseic acid
Catalog No.:BCX1729
CAS No.:24394-14-7
- 1α, 2α-diacetoxy-8β-isobutanoyloxy-9α-benzoyloxy-15-β-(β-furancarbonyloxy)-4β, 6β-dihydroxy-β-dihydr...
Catalog No.:BCX1728
CAS No.:2170562-63-5
- Arabidopyl alcohol
Catalog No.:BCX1727
CAS No.:1400234-92-5
- Celangulin XIX
Catalog No.:BCX1726
CAS No.:403505-80-6
- Angulatin B
Catalog No.:BCX1725
CAS No.:142546-07-4
- 2',3'-Dihydro-2'-hydroxyprotoapigenone
Catalog No.:BCX1724
CAS No.:1365655-88-4
- 2R-3',4',8-Trihydroxyflavanone-7-O-glucoside
Catalog No.:BCX1723
CAS No.:56389-87-8
- Ejaponine A
Catalog No.:BCX1736
CAS No.:1253119-28-6
- Celangulin
Catalog No.:BCX1737
CAS No.:116159-73-0
- Dihydrodehydrodiconiferyl alcohol 9-O-α-L-rhamnopyranoside
Catalog No.:BCX1738
CAS No.:1252572-36-3
- Dihydrodehydrodiconiferyl alcohol 9-O-β-D-xylopyranoside
Catalog No.:BCX1739
CAS No.:1015175-06-0
- 1α-Hydroxy-3-deoxypseudoanisatin
Catalog No.:BCX1740
CAS No.:258854-65-8
- Propionic acid (9R,10R)-9-acetoxy-8,8-dimethyl-9,10-dihydro-2H,8H-benzo[1,2-b:3,4- b′]dipyran-2-one-...
Catalog No.:BCX1741
CAS No.:440094-34-8
- (8R,8'R)-Matairesinol 4,4'-di-O-β-D-glucopyranoside
Catalog No.:BCX1742
CAS No.:41948-08-7
- Nortrachelogenin 4'-O-β-gentiobioside
Catalog No.:BCX1743
CAS No.:1961246-09-2
- Ophiopogonin R
Catalog No.:BCX1744
CAS No.:1418183-25-1
- Qianhucoumarin D
Catalog No.:BCX1745
CAS No.:20516-19-2
- Peucedanol
Catalog No.:BCX1746
CAS No.:46992-81-8
- Oxyresveratrol 4'-O-β-D-glucopyranoside
Catalog No.:BCX1747
CAS No.:863329-68-4
Alkaloids from Piper nigrum Exhibit Antiinflammatory Activity via Activating the Nrf2/HO-1 Pathway.[Pubmed:28185326]
Phytother Res. 2017 Apr;31(4):663-670.
In the present study, ten alkaloids, namely chabamide (1), pellitorine (2), Retrofractamide A (3), pyrroperine (4), isopiperolein B (5), piperamide C9:1 (8E) (6), 6,7-dehydrobrachyamide B (7), 4,5-dihydropiperine (8), dehydropipernonaline (9), and piperine (10), were isolated from the fruits of Piper nigrum. Among these, chabamide (1), pellitorine (2), Retrofractamide A (3), isopiperolein B (5), and 6,7-dehydrobrachyamide B (7) exhibited significant inhibitory activity on lipopolysaccharide-induced nitric oxide (NO) production in RAW264.7 cells, with IC(50) values of 6.8, 14.5, 30.2, 23.7, and 38.5 muM, respectively. Furthermore, compound 1 inhibited lipopolysaccharide-induced NO production in bone marrow-derived macrophages with IC(50) value of 9.5 muM. Consistent with NO inhibition, treatment of RAW264.7 cells with chabamide (1), pellitorine (2), and 6,7-dehydrobrachyamide B (7) suppressed expression of inducible NO synthase and cyclooxygenase-2. Chabamide (1), pellitorine (2), and 6,7-dehydrobrachyamide B (7) induced heme-oxygenase-1 expression at the transcriptional level. In addition, compound 1 induced the nuclear translocation of nuclear factor-E2-related factor 2 (Nrf2) and upregulated the expression of Nrf2 target genes, NAD(P)H:quinone oxidoreductase 1 and gamma-glutamyl cysteine synthetase catalytic subunit, in a concentration-dependent manner in RAW264.7 cells. These findings suggest that chabamide (1) from P. nigrum exert antiinflammatory effects via the activation of the Nrf2/heme-oxygenase-1 pathway; hence, it might be a promising candidate for the treatment of inflammatory diseases. Copyright (c) 2017 John Wiley & Sons, Ltd.
Search for new type of PPARgamma agonist-like anti-diabetic compounds from medicinal plants.[Pubmed:24882400]
Biol Pharm Bull. 2014;37(6):884-91.
Potent ligands of peroxisome proliferator-activated receptor gamma (PPARgamma) such as thiazolidinediones (pioglitazone, troglitazone, etc.) improve insulin sensitivity by increasing the levels of adiponectin, an important adipocytokine associated with insulin sensitivity in adipose tissue. Several constituents from medicinal plants were recently reported to show PPARgamma agonist-like activity in 3T3-L1 cells, but did not show agonistic activity at the receptor site different from thiazolidinediones. Our recent studies on PPARgamma agonist-like constituents, such as hydrangenol and hydrangeic acid from the processed leaves of Hydrangea macrophylla var. thunbergii, piperlonguminine and Retrofractamide A from the fruit of Piper chaba, and tetramethylkaempferol and pentamethylquercetin from the rhizomes of Kaempferia parviflora, are reviewed.
Adipogenetic effects of retrofractamide A derivatives in 3T3-L1 cells.[Pubmed:23910984]
Bioorg Med Chem Lett. 2013 Sep 1;23(17):4813-6.
In a previous study, Retrofractamide A from the fruit of Piper chaba was shown to promote adipogenesis in 3T3-L1 cells. In the present study, Retrofractamide A and its derivatives were synthesized, and their adipogenetic effects in 3T3-L1 cells were examined. Among the tested compounds, an amide composed of 9-(3',4'-methylenedioxyphenyl)-nona-2E,4E,8E-trienoic acid and an n-butyl or n-pentyl amine showed strongest activity. Moreover, the amide with the n-pentyl amine moiety significantly increased the uptake of 2-deoxyglucose into the cells, and also increased the mRNA levels of adiponectin, peroxisome proliferator-activated receptor gamma2 (PPARgamma2), glucose transporter 4 (GLUT4), fatty acid-binding protein (aP2), and CCAAT/enhancer-binding protein (C/EBP) alpha and beta in a similar manner as the PPARgamma agonist troglitazone, although it had less agonistic activity against PPARgamma.
Adipogenic effects of piperlonguminine in 3T3-L1 cells and plasma concentrations of several amide constituents from Piper chaba extracts after treatment of mice.[Pubmed:23584920]
J Nat Med. 2014 Jan;68(1):74-82.
In our previous study, piperlonguminine from the fruit of Piper chaba was reported to promote adipogenesis in 3T3-L1 cells like the peroxisome proliferator-activated receptor-gamma (PPARgamma) agonist, troglitazone. In the present study, the mode of action of piperlonguminine in cells was examined. Piperlonguminine increased mRNA levels of adiponectin, glucose transporter 4, and fatty acid-binding protein (aP2). It also increased mRNA levels of PPARgamma2 but, unlike troglitazone, piperlonguminine did not activate PPARgamma directly in a nuclear receptor cofactor assay. Analyses of plasma from mice treated with piperlonguminine, piperine, and Retrofractamide A, and an extract of the fruit, showed that concentrations of piperlonguminine were higher than those of piperine and Retrofractamide A, and that the "area-under-the-curve" of piperine increased following in vivo administration of the extract.
Drug design for neuropathic pain regulation from traditional Chinese medicine.[Pubmed:23378894]
Sci Rep. 2013;3:844.
FAAH-like anandamide transporter (FLAT) regulates anandamide transport for hydrolysis and may be an attractive drug target for pain regulation. We aimed to discover potential FLAT antagonists from traditional Chinese medicine (TCM) using virtual screening, ligand-based drug design and molecular dynamics simulation (MD). Guineensine and Retrofractamide A exhibited high Dock Scores in FLAT. Consensus from multiple linear regression (MLR; R(2) = 08973) and support vector machine (SVM; R(2) = 0.7988) showed similar bioactivities for Guineensine and the FAAH-1 inhibitor (9Z)-1-(5-pyridin-2-yl-1,3,4-oxadiazol-2-yl)octadec-9-en-1-one. Contour of Guineensine to CoMFA and CoMSIA features also imply bioactivity. MD revealed shake or vibration in the secondary structure of FLAT complexed with Guineensine and (9Z)-1-(5-pyridin-2-yl-1,3,4-oxadiazol-2-yl)octadec-9-en-1-one. Ligand movement might contribute to protein changes leading to vibration patterns. Violent vibrations leading to an overall decrease in FLAT function could be the underlying mechanism for Guineensine. Here we suggest Guineensine as a drug-like compound with potential application in relieving neuropathic pain by inhibiting FLAT.
Insecticidal activity of isobutylamides derived from Piper nigrum against adult of two mosquito species, Culex pipiens pallens and Aedes aegypti.[Pubmed:22010905]
Nat Prod Res. 2012;26(22):2129-31.
The insecticidal activity of Piper nigrum fruit-derived piperidine alkaloid (piperine) and N-isobutylamide alkaloids (pellitorine, guineensine, pipercide and Retrofractamide A) against female adults of Culex pipiens pallens and Aedes aegypti was examined. On the basis of 24-h LD(50) values, the compound most toxic to female C. pipiens pallens was pellitorine (0.4 microg/female symbol) followed by guineensine (1.9 microg/female symbol), Retrofractamide A (2.4 microg/female symbol) and pipercide (3.2 microg/female symbol). LD(50) value of chlorpyrifos was 0.03 microg/female symbol. Against female A. aegypti, the insecticidal activity was more pronounced in pellitorine (0.17 microg/female symbol) than in Retrofractamide A (1.5 microg/female symbol), guineensine (1.7 microg/female symbol), and pipercide (2.0 microg/female symbol). LD(50) value of chlorpyrifos was 0.0014 microg/female symbol.
Effects of amide constituents from pepper on adipogenesis in 3T3-L1 cells.[Pubmed:18477507]
Bioorg Med Chem Lett. 2008 Jun 1;18(11):3272-7.
Several amide constituents (piperlonguminine and retrofractamides A, B, and C) from the fruit of Piper chaba promoted adipogenesis of 3T3-L1 cells. Among them, Retrofractamide A was the most active and significantly increased the amount of adiponectin released into the medium and the uptake of 2-deoxyglucose into the cells. Retrofractamide A also increased mRNA levels of adiponectin, peroxisome proliferator-activated receptor gamma2 (PPARgamma2), glucose transporter 4 (GLUT4), and insulin receptor substrate 1 (IRS-1), but did not act as a PPARgamma agonist different from troglitazone.
ACAT inhibition of alkamides identified in the fruits of Piper nigrum.[Pubmed:17188313]
Phytochemistry. 2007 Mar;68(6):899-903.
In this study, via a bioactivity-guided fractionation of MeOH extracts of the fruits of Piper nigrum, alkamide (5) and five previously-identified alkamides were isolated. Their structures were elucidated via spectroscopic analysis ((1)H, (13)C NMR and ESI-MS), as follows: Retrofractamide A (1), pipercide (2), piperchabamide D (3), pellitorin (4), dehydroretrofractamide C (5) and dehydropipernonaline (6). The IC(50) values determined for the compounds were 24.5 (1), 3.7 (2), 13.5 (3), 40.5 (4), 60 (5) and 90 microM (6), according to the results of an ACAT enzyme assay system using rat liver microsomes. These compounds all inhibited cholesterol esterification in HepG2 cells.
Larvicidal activity of isobutylamides identified in Piper nigrum fruits against three mosquito species.[Pubmed:11902925]
J Agric Food Chem. 2002 Mar 27;50(7):1866-70.
The insecticidal activity of materials derived from the fruits of Piper nigrum against third instar larvae of Culex pipiens pallens, Aedes aegypti, and A. togoi was examined and compared with that of commercially available piperine, a known insecticidal compound from Piper species. The biologically active constituents of P. nigrum fruits were characterized as the isobutylamide alkaloids pellitorine, guineensine, pipercide, and Retrofractamide A by spectroscopic analysis. Retrofractamide A was isolated from P. nigrum fruits as a new insecticidal principle. On the basis of 48-h LC(50) values, the compound most toxic to C. pipiens pallens larvae was pipercide (0.004 ppm) followed by Retrofractamide A (0.028 ppm), guineensine (0.17 ppm), and pellitorine (0.86 ppm). Piperine (3.21 ppm) was least toxic. Against A. aegypti larvae, larvicidal activity was more pronounced in Retrofractamide A (0.039 ppm) than in pipercide (0.1 ppm), guineensine (0.89 ppm), and pellitorine (0.92 ppm). Piperine (5.1 ppm) was relatively ineffective. Against A. togoi larvae, Retrofractamide A (0.01 ppm) was much more effective, compared with pipercide (0.26 ppm), pellitorine (0.71 ppm), and guineensine (0.75 ppm). Again, very low activity was observed with piperine (4.6 ppm). Structure-activity relationships indicate that the N-isobutylamine moiety might play a crucial role in the larvicidal activity, but the methylenedioxyphenyl moiety does not appear essential for toxicity. Naturally occurring Piper fruit-derived compounds merit further study as potential mosquito larval control agents or as lead compounds.


