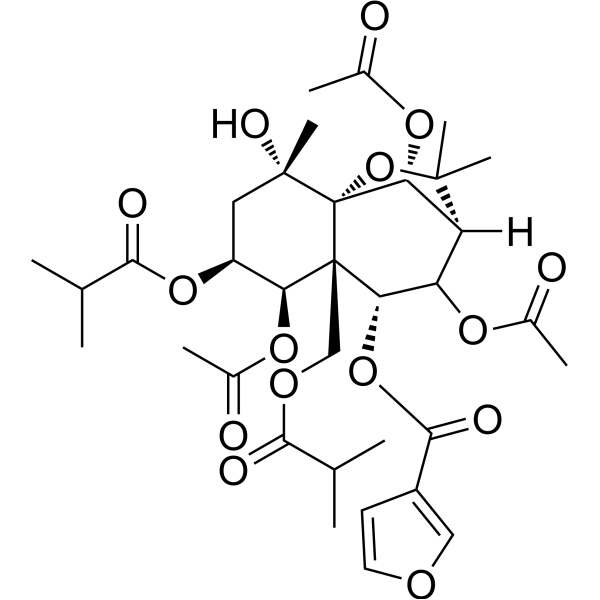
Angulatin BCAS# 142546-07-4 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 142546-07-4 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C34H46O15 | M.Wt | 694.72 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Angulatin B Dilution Calculator

Angulatin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4394 mL | 7.1971 mL | 14.3943 mL | 28.7886 mL | 35.9857 mL |
| 5 mM | 0.2879 mL | 1.4394 mL | 2.8789 mL | 5.7577 mL | 7.1971 mL |
| 10 mM | 0.1439 mL | 0.7197 mL | 1.4394 mL | 2.8789 mL | 3.5986 mL |
| 50 mM | 0.0288 mL | 0.1439 mL | 0.2879 mL | 0.5758 mL | 0.7197 mL |
| 100 mM | 0.0144 mL | 0.072 mL | 0.1439 mL | 0.2879 mL | 0.3599 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2',3'-Dihydro-2'-hydroxyprotoapigenone
Catalog No.:BCX1724
CAS No.:1365655-88-4
- 2R-3',4',8-Trihydroxyflavanone-7-O-glucoside
Catalog No.:BCX1723
CAS No.:56389-87-8
- Polyporoid C
Catalog No.:BCX1722
CAS No.:1042362-47-9
- Polyporoid B
Catalog No.:BCX1721
CAS No.:1042362-45-7
- Maritimetin
Catalog No.:BCX1720
CAS No.:576-02-3
- Aristol-1(10)-en-9-ol
Catalog No.:BCX1719
CAS No.:114339-94-5
- (2E,4E)-8-Hydroxy-2,7-dimethyl-decadien-(2,4)-disaeure-(1,10)-dioic acid
Catalog No.:BCX1718
CAS No.:2808401-10-5
- Angulatin K
Catalog No.:BCX1717
CAS No.:1631992-80-7
- Rabdophyllin G
Catalog No.:BCX1716
CAS No.:82460-75-1
- Wallichoside
Catalog No.:BCX1715
CAS No.:60657-36-5
- 2,3-Dihydro-3-hydroxy-2-oxo-1H-indole-3-acetic acid
Catalog No.:BCX1714
CAS No.:57061-17-3
- Protoapigenin
Catalog No.:BCX1713
CAS No.:879325-07-2
- Celangulin XIX
Catalog No.:BCX1726
CAS No.:403505-80-6
- Arabidopyl alcohol
Catalog No.:BCX1727
CAS No.:1400234-92-5
- 1α, 2α-diacetoxy-8β-isobutanoyloxy-9α-benzoyloxy-15-β-(β-furancarbonyloxy)-4β, 6β-dihydroxy-β-dihydr...
Catalog No.:BCX1728
CAS No.:2170562-63-5
- Phaseic acid
Catalog No.:BCX1729
CAS No.:24394-14-7
- (2E,4E)-2,4-Decadienoic acid
Catalog No.:BCX1730
CAS No.:30361-33-2
- Isochavicine
Catalog No.:BCX1731
CAS No.:30511-77-4
- Piperundecalidine
Catalog No.:BCX1732
CAS No.:88660-11-1
- 15α-Hydroxy-20-oxo-6,7-seco-ent-kaur-16-en-1,7α(6,11α)-diolide
Catalog No.:BCX1733
CAS No.:1355450-84-8
- Celangulatin D
Catalog No.:BCX1734
CAS No.:1000784-45-1
- Retrofractamide A
Catalog No.:BCX1735
CAS No.:94079-67-1
- Ejaponine A
Catalog No.:BCX1736
CAS No.:1253119-28-6
- Celangulin
Catalog No.:BCX1737
CAS No.:116159-73-0
Two new sesquiterpene polyol esters from the root barks of Celastrus angulatus.[Pubmed:21462033]
J Asian Nat Prod Res. 2011 Apr;13(4):304-11.
Angulatin F (1) and angulatin I (2), two new sesquiterpene polyol esters, were isolated from the root barks of Celastrus angulatus, together with six known compounds 1beta,2beta-diacetoxy-4alpha,6alpha-dihydroxy-8alpha-isobutanoyloxy-9beta-benzoyloxy-15-(alpha-methyl) butanoyloxy-beta-dihydroagrofuran (3), angulatin A (4), Angulatin B (5), celangulatin E (6), 1beta,2beta,15-triacetoxy-4alpha,6alpha-dihydroxy-8alpha-isobutanoyloxy-9beta-benzoyloxy-beta-dihydroagrofuran (7), and celangulin I (8). The structures of 1 and 2 were elucidated as 1beta,2beta,6alpha,15-tetraacetoxy-4alpha-hydroxy-8beta,9alpha-difuroyloxy-beta-dihydroagrofuran and 1beta,2beta,6alpha,8beta,15-pentaacetoxy-4alpha-hydroxy-9beta-furoyloxy-beta-dihydroagrofuran by spectroscopic means.
[Assignments of 1HNMR fingerprint of the root bark of Celastrus angulatus].[Pubmed:12585134]
Yao Xue Xue Bao. 2001 Jun;36(6):462-6.
AIM: To assign the 1HNMR finger-print of the root bark of Celastrus angulatus. METHODS: Silica gel column chromatography was used to separate the chemical constituents of CGE A of the root bark of C. angulatus. The characteristic signals of the 1HNMR finger-print were assigned after determining the structures of the compounds isolated from CGE A. RESULTS: 1HNMR finger-prints of the samples of C. angulatus collected from different regions showed highly characteristic features and reproducibility. Three compounds predominant in CGE A were isolated and their structures were determined by spectral analysis as: angulatin A (1), Angulatin B (2) and angulatin C (3). CONCLUSION: Compound 3 is a new compound. Compound 2 was isolated from C. angulatus for the first time. The 1HNMR finger-print of CGE A of the root bark of C. angulatus showed mainly the characteristic signals of the above three compounds and might be utilized for the original authentication of this plant.


