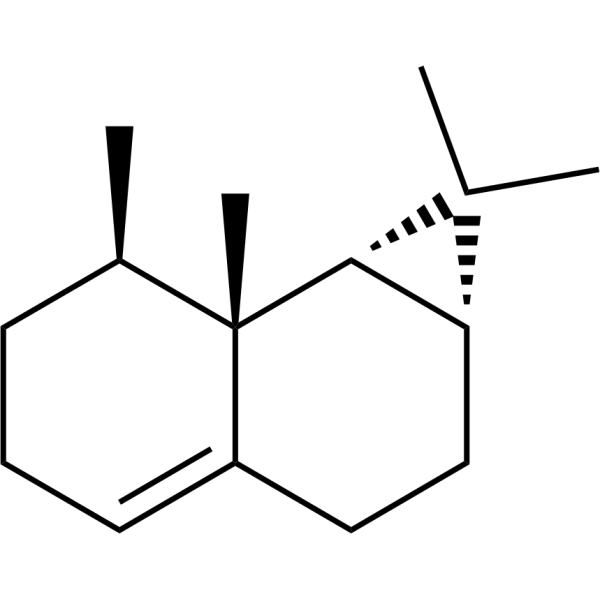
CalareneCAS# 17334-55-3 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 17334-55-3 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C15H24 | M.Wt | 204.35 |
| Type of Compound | Other Terpenoids | Storage | Desiccate at -20°C |
| Synonyms | (+)-1(10)-Aristolene,(+)-Calarene | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Calarene Dilution Calculator

Calarene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8936 mL | 24.4678 mL | 48.9356 mL | 97.8713 mL | 122.3391 mL |
| 5 mM | 0.9787 mL | 4.8936 mL | 9.7871 mL | 19.5743 mL | 24.4678 mL |
| 10 mM | 0.4894 mL | 2.4468 mL | 4.8936 mL | 9.7871 mL | 12.2339 mL |
| 50 mM | 0.0979 mL | 0.4894 mL | 0.9787 mL | 1.9574 mL | 2.4468 mL |
| 100 mM | 0.0489 mL | 0.2447 mL | 0.4894 mL | 0.9787 mL | 1.2234 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- β-Maaliene
Catalog No.:BCX1841
CAS No.:489-29-2
- Heteroclitin Ib
Catalog No.:BCX1840
CAS No.:1011762-96-1
- Casuarinin
Catalog No.:BCX1839
CAS No.:79786-01-9
- 23-O-β-D-Glucopyranosyl-20-isoveratramine
Catalog No.:BCX1838
CAS No.:148440-62-4
- Kadsurenin C
Catalog No.:BCX1837
CAS No.:145722-88-9
- (+)-Osbeckic acid
Catalog No.:BCX1836
CAS No.:112923-64-5
- Angeloylbinankadsurin A
Catalog No.:BCX1835
CAS No.:77165-80-1
- Schiarisanrin C
Catalog No.:BCX1834
CAS No.:130252-44-7
- Interiorin D
Catalog No.:BCX1833
CAS No.:133360-40-4
- Elephanoside D
Catalog No.:BCX1832
CAS No.:1005340-21-5
- Schisanlignone B
Catalog No.:BCX1831
CAS No.:135459-86-8
- Schiarisanrin E
Catalog No.:BCX1830
CAS No.:697228-90-3
- Interiorin C
Catalog No.:BCX1843
CAS No.:133360-39-1
- Campneoside I
Catalog No.:BCX1844
CAS No.:95519-12-3
- Valerena-4,7(11)-diene
Catalog No.:BCX1845
CAS No.:351222-66-7
- (7R)-Isocampneoside I
Catalog No.:BCX1846
CAS No.:1119034-95-5
- Cynanoside J
Catalog No.:BCX1847
CAS No.:1800029-53-1
- Stauntoside R
Catalog No.:BCX1848
CAS No.:2172321-58-1
- Heteroclitin E
Catalog No.:BCX1849
CAS No.:140369-77-3
- Longipedunin A
Catalog No.:BCX1850
CAS No.:886441-72-1
- Heteroclitin G
Catalog No.:BCX1851
CAS No.:144027-74-7
- Schiarisanrin B
Catalog No.:BCX1852
CAS No.:130252-43-6
- Cynatratoside D
Catalog No.:BCX1853
CAS No.:97399-99-0
- Interiotherin D
Catalog No.:BCX1854
CAS No.:460090-66-8
Assessing the Larvicidal Properties of Endemic Campeche, Mexico Plant Piper cordoncillo var. apazoteanum (Piperaceae) against Aedes aegypti (Diptera: Culicidae) Mosquitoes.[Pubmed:37103127]
Insects. 2023 Mar 24;14(4):312.
The research aims to investigate the mortality effect of essential oil from Piper cordoncillo var. apazoteanum, an endemic plant from Campeche, Mexico, on early second-instar Aedes aegypti larvae; it also aims to identify the volatile compounds present in the fresh leaves of the plant. To test the effectiveness of the essential oil, we followed World Health Organization Standard Procedures. Larvae were observed for 17 consecutive days after treatment to determine the mortality and growth-inhibitory effect exerted by the essential oil. The results showed that the essential oil was effective in controlling mosquito populations. At a concentration of 800 ppm, the oil achieved an effectiveness rate of 70.00 +/- 8.16% after 24 h, increasing to 100.00 +/- 0.01% mortality after 72 h. With a concentration of 400 ppm, the effectiveness was 98.33 +/- 0.17% by the end of the experiment. Furthermore, the obtained results demonstrated that the LC(50) value was 61.84 +/- 6.79 ppm, while the LC(90) value was 167.20 +/- 11.49 ppm. Essential oil concentrations inhibited the growth of immature insect stages, with concentrations between 800-100 ppm demonstrating very high inhibitory activity, and the lowest concentration of 50 ppm showing high inhibitory activity. The study also identified 24 chemical compounds representing 86.71% of the volatile compound composition of the fresh leaves of P. cordoncillo; the most abundant compounds were Safrole, Caryophyllene oxide, E-Nerolidol, and Calarene epoxide. The method used to extract the volatile compounds, solvent-free microwave extraction (SFME), is a promising alternative to traditional methods that avoids the use of potentially harmful solvents, making it more ecologically friendly and potentially safer for professionals handling the extracted compounds. Overall, the study demonstrates the potential of P. cordoncillo essential oil as an effective means of controlling mosquito populations, and provides valuable information on the chemical composition of the plant.Moreover, our study is the first to report on the biological activity and chemical composition of P. cordoncillo worldwide.
Comparative Analysis of the Floral Fragrance Compounds of Panax notoginseng Flowers under the Panax notoginseng-pinus Agroforestry System Using SPME-GC-MS.[Pubmed:35684502]
Molecules. 2022 Jun 1;27(11):3565.
Panax notoginseng is a medicinal plant in China, the flowers of which have high medicinal value. To study the differences in the floral fragrance compounds of P. notoginseng flowers (bionic wild cultivation) from the forests of Yunnan Province, the floral fragrance compounds from four varieties of P. notoginseng flowers (four-forked seven leaves, three-forked seven leaves, four-forked five-seven leaves, and three-forked five-six leaves) were compared and analyzed via headspace solid phase microextraction combined with gas chromatography-mass spectrometry methods. A total of 53 floral fragrance compounds from the P. notoginseng flowers were divided into eight categories, mainly consisting of terpenes, alkynes, aromatic hydrocarbons, and alcohols. Moreover, high contents of 3-carene, germacrene D, (-)-alpha-gurjunene, valencene, (+)-gamma-gurjunene, menogene, and aromandendrene were identified from the flowers of different P. notoginseng varieties. Interestingly, floral fragrance compounds such as 3-carene, valencene, aromandendrene, menogene, and (+)-gamma-gurjunene were first reported in the flowers of P. notoginseng. Cluster analysis showed that P. notoginseng with four-forked and three-forked leaves clustered into two subgroups, respectively. In addition, principal component analysis showed that (+)-gamma-gurjunene, (+)-Calarene, copaene, 1,8,12-bisabolatriene, gamma-elemene, (-)-aristolene, caryophyllene, 3-carenes, and 2,6-dimethyl-1,3,6-heptatriene can be used to distinguish the floral fragrance components of four P. notoginseng flower species. This study provides a theoretical basis for elucidating the floral fragrance compounds emitted from the flowers of different P. notoginseng varieties in an agroforestry system.
Identification of volatile active components in Acori Tatarinowii Rhizome essential oil from different regions in China by C6 glioma cells.[Pubmed:32807141]
BMC Complement Med Ther. 2020 Aug 17;20(1):255.
BACKGROUND: Acori Tatarinowii Rhizome (ATR) is a well-recognized Chinese herbal medicine prescribed to treat neurological disorders. The essential oil (ATEO) is considered as the active fraction of ATR and the content of ATEO is used as the only indicator for ATR content determination. The quality of ATEO varies widely due to region difference; however, little is known about how to study ATEO quality chemically and biologically in response to region difference. Thus, it is of great importance to identify volatile active components in ATEO to conduct quality study. In this study, we analyzed ATEO from different regions in China using chemical component analysis combined with biological activity evaluation. METHODS: GC-MS was used to obtain different volatile component profiles of ATEO and significantly changed volatile components were screened out. The neuroprotective activities of ATEO, including anti-oxidation, anti-inflammation and neurotrophic functions, were revealed in C6 glioma cells. The correlation study between the bioactivities and the components was performed. RESULTS: 57 volatile components, including terpenoids, phenylpropanoids, aromatic compounds, and other aliphatic compounds, were identified. 8 volatile components (beta-asarone, cis-methyl isoeugenol, gamma-asarone, methyleugenol, Calarene, longifolene, beta-caryophyllene and caryophyllene oxide) from ATEO were significantly changed due to region difference and 2 of them (beta-asarone and gamma-asarone) showed strong correlation with neuroprotective activities. CONCLUSIONS: Our results reveal that ATEO from different regions in China show great changes in chemical composition and biological activity. Moreover, phenylpropanoids (beta-asarone and gamma-asarone) present strong correlation with the bioactivities, which are considered as volatile active components in ATEO. The findings will be useful for the development of quality study of ATEO.
Essential oil of the malagasy grass Elionurus tristis Hack. contains several undescribed sesquiterpenoids.[Pubmed:30851508]
Phytochemistry. 2019 Jun;162:29-38.
Essential oils (EOs) obtained from aerial parts and roots of Elionurus tristis were investigated by GC, GC-MS, pc-GC and NMR. Both aerial parts and roots EOs contained common molecules such as alpha-pinene, camphene, trans-alpha-bergamotene and Calarene. Moreover, we identified several unusual sesquiterpenes and four undescribed compounds, 7-epi-khusian-2-ol, 4,8-di-epi-acorone, 2-epi-ziza-5-en-2-ol and antsorenone. The last one exhibits an undescribed natural sesquiterpene skeleton. All undescribed compounds were isolated and fully characterized by MS, 1D and 2D-NMR. Furthermore, the formation pathway of the Antsorane skeleton is discussed.
Chemical Composition, Anticancer, Anti-neuroinflammatory, and Antioxidant Activities of the Essential Oil of Patrinia scabiosaefolia.[Pubmed:27586471]
Chin J Integr Med. 2018 Mar;24(3):207-212.
OBJECTIVE: To study the chemical composition, anticancer, anti-neuroinflflammatory, and antioxidant activities of the essential oil of Patrinia scabiosaefolia (EO-PS). METHODS: Patrinia scabiosaefolia was analyzed by gas chromatography-mass spectrometry. Eight human carcinoma cell lines, including SGC-7901, AGS, HepG2, HT-29, HCT-8, 5-FU/HCT-8, HeLa, and MDA-MB-231, were assessed by methylthiazolyldiphenyltetrazolium bromide (MTT) assay. Anti-neuroinflflammatory activity was assessed by production of interleukin (IL)-1beta and IL-6 induced by lipopolysaccharide in BV-2 cells (microglia from mice). The antioxidant activity was evaluated with a 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging assay. RESULTS: Forty-four components, representing 83.919% of the total oil, were identifified in the EO-PS. The major constituents were caryophyllene oxide (12.802%), caryophyllene (6.909%), alpha-caryophyllene (2.927%), beta-damascenone (3.435%), Calarene (5.621%), and phenol (3.044%). The MTT assay showed that the EO-PS exhibited significant dose-dependent growth inhibition in the 50-200 mug/mL dilution range. The EO-PS exhibited a dose-dependent scavenging activity against the DPPH radical, with an half of maximal inhibitory concentration 1.455 mg/mL. CONCLUSIONS: The EO-PS possesses a wide range of antitumor, anti-neuroinflflammatory and antioxidant activities, suggesting that it may be a good candidate for further investigations of new bioactive substances.
delta-Cadinene, Calarene and .delta-4-Carene from Kadsura heteroclita Essential Oil as Novel Larvicides Against Malaria, Dengue and Filariasis Mosquitoes.[Pubmed:27151483]
Comb Chem High Throughput Screen. 2016;19(7):565-71.
Mosquitoes (Diptera: Culicidae) are major vectors of important pathogens and parasites. Malaria, dengue fever, yellow fever, filariasis, schistosomiasis and Japanese encephalitis cause millions of deaths every year. Mosquito control is being challenging due to the development of pesticide resistance and negative environmental concerns. In this scenario, plants employed in traditional Asian medicine may be alternative sources of newer and effective mosquitocides. In this research, we evaluated the larvicidal activity of Kadsura heteroclita leaf essential oil (EO) and its major chemical constituents (delta-Cadinene, Calarene and delta-4-Carene) against the malaria vector Anopheles stephensi, the dengue vector Aedes aegypti and the filariasis vector Culex quinquefasciatus. The chemical composition of the EO was analyzed by gas chromatography-mass spectroscopy. GC-MS revealed that the essential oil of K. heteroclita contained 33 compounds. The major chemical components were delta-Cadinene (18.3%), Calarene (14.8%) and delta-4-Carene (12.5%). The EO had a significant toxic effect against early third-stage larvae of An. stephensi, Ae. aegypti and Cx. quinquefasciatus, with LC50 values of 102.86, 111.79 and 121.97 microg/mL. The three major constituents extracted from the K. heteroclita EO were tested individually for acute toxicity against larvae of the three mosquito vectors. delta-Cadinene, Calarene and delta-4-Carene appeared most effective against An. stephensi (LC50 = 8.23, 12.34 and 16.37 microg/mL, r espectively) followed by Ae. aegypti (LC50 = 9.03, 13.33 and 17.91 microg/mL), and Cx. quinquefasciatus (LC50; = 9.86, 14.49 and 19.50 microg/mL). Overall, this study adds knowledge to develop newer and safer natural larvicides against malaria, dengue and filariasis mosquito.
[Visualization for Traditional Quality Management Techniques--Characterization Method for Spikenard of INUBUSHI SEIYAKU Established in the Edo Period].[Pubmed:26427103]
Yakushigaku Zasshi. 2015;50(1):89-93.
INUBUSHI SEIYAKU, a Japanese pharmaceutical company established in 1807, manufactures KEISHIN-TAN. This is an original drug developed by the company, and consists of 14 exotic natural medicines, spikenard, oriental bezoar, musk, agarwood, etc. It has been used for adjusting the autonomic nervous system and physical conditions. We studied the original methods of the traditional quality management techniques handed down within INUBUSHI SEIYAKU in selecting the appropriate spikenard (Nardostachys chinensis) for medicinal use. Currently, spikenards are mainly used as incense rather than medicine. KEISHIN-TAN is a rare case in that the bulk powder of the spikenards is used for pharmaceutical products in Japan. We examined the morphological characteristics and made an analysis of the component of spikenards selected by traditional methods. The raw material of the spikenards was purchased from the Japanese market, and was classified into two categories-superior, fit for medicinal use and defective, to be discarded-by traditional methods of INUBUSHI SEIYAKU. The methods of the characterization of the spikenard by INUBUSHI SEIYAKU were investigated. As a result, only thick spikenard roots over 2.0 cm in length and approximately 0.5 cm in diameter were found to be used, and the total weight of the superior was only 15% of the raw material. By comparing the weights of hexane extracts and GC-MS analyses, the content of Calarene--main sedative compound in spikenards--in the superior material was 2.8 times higher than the raw material and 4.3 times higher than the defective material. The ways to devise how to enhance the pharmacological effects of spikenards may be contained in this method. These results revealed the traditional spikenard selection criteria, and may show the indications of using spikenard or its compounds for medicinal purposes.
Chemical Composition of Nardostachys grandiflora Rhizome Oil from Nepal--A Contribution to the Chemotaxonomy and Bioactivity of Nardostachys.[Pubmed:26197553]
Nat Prod Commun. 2015 Jun;10(6):1067-70.
The essential oil from the dried rhizome of Nardostachys grandiflora, collected from Jaljale, Nepal, was obtained in 1.4% yield, and a total of 72 compounds were identified constituting 93.8% of the essential oil. The rhizome essential oil of N. grandiflora was mostly composed of Calarene (9.4%), valerena-4,7(11)-diene (7.1%), nardol A (6.0%), 1(10)-aristolen-9beta-ol (11.6%), jatamansone (7.9%), valeranal (5.6%), and cis-valerinic acid (5.7%). The chemical composition of N. grandiflora rhizome oil from Nepal is qualitatively very different than those from Indian, Chinese, and Pakistani Nardostachys essential oils. In this study we have evaluated the chemical composition and biological activities of N. grandiflora from Nepal. Additionally, 1(10)-aristolen-9beta-ol was isolated and the structure determined by NMR, and represents the first report of this compound from N. grandiflora. N. grandiflora rhizome oil showed in-vitro antimicrobial activity against Bacillus cereus, Escherichia coli, and Candida albicans (MIC = 156 mug/mL), as well as in-vitro cytotoxic activity on MCF-7 cells.
Essential oil composition of valeriana jatamansi jones from himalayan regions of India.[Pubmed:26009656]
Indian J Pharm Sci. 2015 Mar-Apr;77(2):218-22.
Valeriana jatamansi Jones germplasm collected from sub-temperate Himalayan region of Uttarakhand and North-East state of Meghalaya, India was evaluated under identical conditions at National Bureau of Plant Genetic Resources, Bhowali, India, to study germplasm diversity based on essential oil composition. Twenty one compounds were identified in V. jatamansi root oil by GC and GC-MS. The major compounds identified were patchouli alcohol (0.4-63.7%), maaliol (2.9-53.8%), seychellene (4.1-27.4%), Calarene/ss-gurjunene (3.0-20.8%), alpha-santalene (0.6-12.0%). Other compounds present were bornyl acetate (0.6-1.5%), alpha-guaiene (0.7-2.3%), alpha-bulnesene/delta-guaiene (0.7-6.3%), 7-epi-alpha-selinene (0.4-1.4%), kessane (2.1-3.3%), spathulenol (0.7-3.4%), viridiflorol (0.9-7.1%), alpha-patchoulene (0.8-6.6%), ss-patchoulene (0.4-0.8%). Two superior chemotypes identified in V. jatamansi oil from Uttarakhand were: patchouli alcohol rich (IC573221, 63.7%) and maaliol rich (IC573222, 53.8%; IC589096, 51.7%), while accession from north-east was patchouli alcohol rich chemotype (IC574522, 57.2%). These superior chemotypes with higher amounts of patchouli alcohol and maaliol could be used for promoting cultivation as well as for meeting need of pharmaceutical industries.
Chemical composition and acetylcholinesterase inhibitory activity of Artemisia maderaspatana essential oil.[Pubmed:25885940]
Pharm Biol. 2015;53(11):1677-83.
CONTEXT: To date, there are no reports to validate the Indian traditional and folklore claims of Artemisia maderaspatana L. (syn. Grangea maderaspatana L.) (Asteraceae) for the treatment of Alzheimer's disease. OBJECTIVE: The present study characterizes the volatile components (non-polar compounds) of A. maderaspatana and evaluates its acetylcholinesterase inhibition potential. MATERIALS AND METHODS: The essential oils (yield 0.06% v/w) were obtained from fresh aerial part of A. maderaspatana. The characterization of volatile components (non-polar compounds) was performed by GC-MS data and with those of reference compounds compiled in the spectral library of in-house database. The in vitro acetylcholinesterase (AChE) inhibition of the volatile organic constituents (VOC's) of A. maderaspatana aerial part was evaluated in varying concentration ranges (0.70-44.75 microg/mL) with Ellman's method. RESULTS: The major components were alpha-humulene (46.3%), beta-caryophyllene (9.3%), alpha-copaene (8.2%), beta-myrcene (4.3%), Z(E)-alpha-farnesene (3.7%), and Calarene (3.5%). Chemical variability among other Artemisia spp. from different climatic regions of India and countries namely Iran and France was observed. The experimental results showed that diverse volatile organic constituents of A. maderaspatana have significant acetylcholinesterase inhibitory activity (an IC50 value of 31.33 +/- 1.03 microg/mL). This is the first report on the inhibition of acetylcholinesterase properties of essential oil of A. maderaspatana obtained from fresh aerial part. CONCLUSIONS: The present results indicate that essential oil of A. maderaspatana isolated from the northern region of India could inhibit AChE moderately. Therefore, the possibility of novel AChE inhibitors might exist in VOCs of this plant.
Effect of roasting on the volatile constituents of Trichosanthes kirilowii seeds.[Pubmed:28911420]
J Food Drug Anal. 2014 Sep;22(3):310-317.
Roasted Trichosanthes kirilowii seeds have much more intense flavor than the raw seeds, and are commonly used as food and in the preparations of many medicinal formulations. Volatile constituents in the raw and roasted T. kirilowii seeds were separated by simultaneous distillation and extraction, and analyzed by gas chromatography-mass spectrometry on two capillary gas chromatography columns of different polarities (DB-WAX and HP-1). A total of 40 volatile compounds were identified in the raw seeds, with pentanal, 2-pentanol, styrene, (Z)-2-heptenal, (+)-Calarene, and alpha-muurolene being the predominant compounds; 40 volatile compounds were also identified in the roasted seeds, with 3-methylbutanal, ethanol, 2-butanol, 2,3-butanediol, (E,E)-2,4-nonadienal, and 2-isopropyl-5-methyl-9-methylene-bicyclo[4.4.0]dec-1-ene being the most abundant compounds. A total of 15 compounds, mostly aldehydes, were common in both seeds. Roasting of T. kirilowii seeds resulted in a significant decrease in the levels of sesquiterpenes and short-chain aliphatic aldehydes. By contrast, high concentrations of 3-methylbutanal, ethanol, 2-butanol, and alkyl pyrazines were generated, which was responsible for the unique flavor of the roasted seeds. The study results may be useful for optimizing the roasting process and oil processing of T. kirilowii seeds.
Characterization of novel single-variety oxygenated sesquiterpenoid hop oil fractions via headspace solid-phase microextraction and gas chromatography-mass spectrometry/olfactometry.[Pubmed:24152289]
J Agric Food Chem. 2013 Nov 6;61(44):10555-64.
The volatile composition of novel varietal oxygenated sesquiterpenoid hop oil fractions ("spicy essences") was characterized by headspace solid-phase microextraction in combination with gas chromatography-mass spectrometry. Oxygenated sesquiterpenes represent the major chemical compound class, accounting for at least 65% of the total volatile fraction. In addition to oxygenated sesquiterpenes, spicy hop essences consist of several ketones, sesquiterpene and monoterpene hydrocarbons, and a relatively high number of unidentified compounds. On the basis of their relative composition, spicy hop essences can be fully differentiated according to their varietal origin. Multidimensional gas chromatography in combination with time-of-flight mass spectrometry on spicy hop essence cv. Spalter Select further demonstrated the enormous complexity of this particular hop oil fraction. The aromagram obtained via gas chromatography-olfactometry comprised nine odor-active regions described in terms of "citrus", "green", "haylike", "earthy", "woody", and "spicy". 2-Undecanone, 2-tridecanone, gamma-cadinene, alpha-calacorene, Calarene, humuladienone, caryolan-1-ol, caryophyllene oxide enantiomers, and humulene epoxide II are tentatively identified in the odor-active zones.
Differences in the volatile compositions of ginseng species (Panax sp.).[Pubmed:22804575]
J Agric Food Chem. 2012 Aug 8;60(31):7616-22.
The volatile compositions in dried white ginseng according to species (Panax ginseng, Panax notoginseng, and Panax quinquefolius) were analyzed and compared by applying multivariate statistical techniques to gas chromatography-mass spectrometry data sets. Main volatile compounds of ginseng species in the present study were sesquiterpenes, such as bicyclogermacrene, (E)-beta-farnesene, beta-panasinsene, Calarene, alpha-humulene, beta-elemene, etc. In particular, alpha-selinene, alpha-terpinolene, beta-bisabolene, beta-phellandrene, beta-sesquiphellandrene, zingiberene, germacrene D, limonene, alpha-gurjunene, (E)-caryophyllene, delta-cadinene, (E)-beta-farnesene, alpha-humulene, bicyclogermacrene, longiborn-8-ene, beta-neoclovene, and (+)-spathulenol were mainly associated with the difference between P. ginseng and P. notoginseng versus P. quinquefolius species. On the other hand, the discrimination between P. ginseng and P. notoginseng could be constructed by hexanal, 2-pyrrolidinone, (E)-2-heptenal, (E)-2-octenal, heptanal, isospathulenol, (E,E)-2,4-decadienal, 3-octen-2-one, benzaldehyde, 2-pentylfuran, and (E)-2-nonenal.
Neolitsea aciculata essential oil inhibits drug-resistant skin pathogen growth and Propionibacterium acnes-induced inflammatory effects of human monocyte leukemia.[Pubmed:21922933]
Nat Prod Commun. 2011 Aug;6(8):1193-8.
This study examined the chemical composition of Neolitsea aciculata essential oil (NAE) and its biological activities. NAE was obtained by hydro-distillation of N. aciculata leaves collected in Jeju Island and analyzed by gas chromatography equipped with a mass spectrometer detector. 1-Dodecen-3-yne (12.5%), Calarene (11.5%) and elemol (9.5%) were identified as the major components of NAE. The antibacterial and anti-inflammatory activities of NAE against skin pathogens were examined to determine the protective properties against acne vulgaris. NAE exhibited moderate to strong antibacterial activity against drug-susceptible and -resistant Propionibacterium acnes and Staphylococcus epidermidis, which are known as acne-causing bacteria. In addition, NAE reduced the P. acnes-induced secretion of tumor necrosis factor-alpha (TNF-alpha) and interleukin-8 (IL-8) in THP-1 cells, highlighting its anti-inflammatory effects. The DPPH radical scavenging activities of NAE also revealed moderate antioxidant properties (IC50, 21.3 microL/mL). Overall, NAE is an attractive candidate as an ingredient in skin care products.


