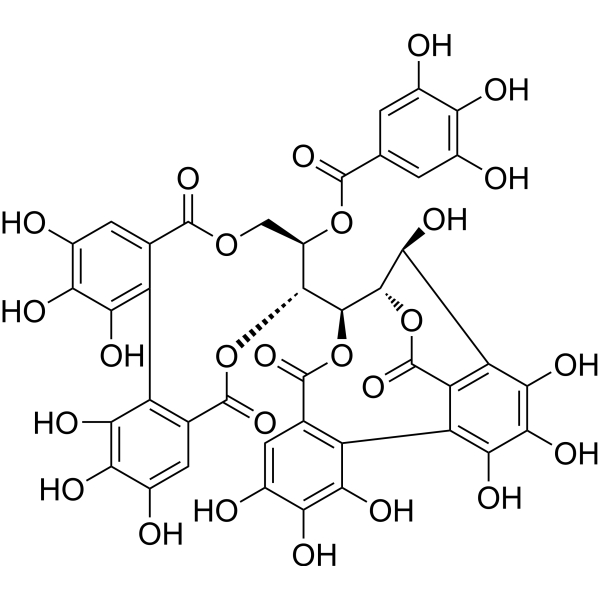
CasuarininCAS# 79786-01-9 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 79786-01-9 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C41H28O26 | M.Wt | 936.65 |
| Type of Compound | Tannins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Casuarinin Dilution Calculator

Casuarinin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0676 mL | 5.3382 mL | 10.6763 mL | 21.3527 mL | 26.6909 mL |
| 5 mM | 0.2135 mL | 1.0676 mL | 2.1353 mL | 4.2705 mL | 5.3382 mL |
| 10 mM | 0.1068 mL | 0.5338 mL | 1.0676 mL | 2.1353 mL | 2.6691 mL |
| 50 mM | 0.0214 mL | 0.1068 mL | 0.2135 mL | 0.4271 mL | 0.5338 mL |
| 100 mM | 0.0107 mL | 0.0534 mL | 0.1068 mL | 0.2135 mL | 0.2669 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 23-O-β-D-Glucopyranosyl-20-isoveratramine
Catalog No.:BCX1838
CAS No.:148440-62-4
- Kadsurenin C
Catalog No.:BCX1837
CAS No.:145722-88-9
- (+)-Osbeckic acid
Catalog No.:BCX1836
CAS No.:112923-64-5
- Angeloylbinankadsurin A
Catalog No.:BCX1835
CAS No.:77165-80-1
- Schiarisanrin C
Catalog No.:BCX1834
CAS No.:130252-44-7
- Interiorin D
Catalog No.:BCX1833
CAS No.:133360-40-4
- Elephanoside D
Catalog No.:BCX1832
CAS No.:1005340-21-5
- Schisanlignone B
Catalog No.:BCX1831
CAS No.:135459-86-8
- Schiarisanrin E
Catalog No.:BCX1830
CAS No.:697228-90-3
- Kadsulignan H
Catalog No.:BCX1829
CAS No.:144049-91-2
- Acetylbinankadsurin A
Catalog No.:BCX1828
CAS No.:77174-33-5
- Kadsuralignan A
Catalog No.:BCX1827
CAS No.:913237-64-6
- Heteroclitin Ib
Catalog No.:BCX1840
CAS No.:1011762-96-1
- β-Maaliene
Catalog No.:BCX1841
CAS No.:489-29-2
- Calarene
Catalog No.:BCX1842
CAS No.:17334-55-3
- Interiorin C
Catalog No.:BCX1843
CAS No.:133360-39-1
- Campneoside I
Catalog No.:BCX1844
CAS No.:95519-12-3
- Valerena-4,7(11)-diene
Catalog No.:BCX1845
CAS No.:351222-66-7
- (7R)-Isocampneoside I
Catalog No.:BCX1846
CAS No.:1119034-95-5
- Cynanoside J
Catalog No.:BCX1847
CAS No.:1800029-53-1
- Stauntoside R
Catalog No.:BCX1848
CAS No.:2172321-58-1
- Heteroclitin E
Catalog No.:BCX1849
CAS No.:140369-77-3
- Longipedunin A
Catalog No.:BCX1850
CAS No.:886441-72-1
- Heteroclitin G
Catalog No.:BCX1851
CAS No.:144027-74-7
Identification and mechanistic investigation of ellagitannins from Osbeckia octandra that attenuate liver fibrosis via the TGF-beta/SMAD signaling pathway.[Pubmed:37580142]
Biosci Biotechnol Biochem. 2023 Oct 25;87(11):1295-1309.
Fibrosis is a major problem in chronic liver disease with limited treatment options due to its complex nature. Herbal medicines are often used as an alternative. The aim of this study was to investigate the therapeutic potential of Osbeckia octandra and to identify its active compounds and regulatory pathways. The effects of crude leaf suspension and boiled leaf extract were investigated in an animal model, and the extract was found to be the more effective treatment. Three major bioactive compounds, pedunculagin, Casuarinin, and gallic acid, were isolated from the extract using the hepatic stellate cell line, LX-2-based antifibrotic effect evaluation system. The results showed that all these compounds ameliorated LX-2 in fibrotic state. This inhibitory mechanism was confirmed through the TGF-beta/SMAD signaling pathway. Collectively, the presence of these compounds in O. octandra suggests its potential as a treatment for liver fibrosis.
Divergent Synthesis of Stachyurin and Casuarinin Focusing on C-Glycosidic Bond Reactivity.[Pubmed:37162021]
Chemistry. 2023 Jul 20;29(41):e202301096.
Stachyurin and Casuarinin are ellagitannins, a class of polyphenols that exhibit various biological activities that have an impact on human health. Casuarinin is a stachyurin stereoisomer. These compounds contain the characteristic C-glycosidic bond between the open-chain d-glucose and the phenol aromatic ring. Therefore, chemical elucidation of the C-glycosidic bond reactivity is required to exploit their multiple bioactivities. This study developed a method for the divergent synthesis of stachyurin and Casuarinin via the alpha-selective C-glycosylation as well as the beta-selective introduction of the oxygen functional group, focusing on structural specificity. The proposed method applies to the syntheses of stachyurin and Casuarinin analogues, thereby facilitating the utilisation of their beneficial bioactivities.
Multitarget Potential of Phytochemicals from Traditional Medicinal Tree, Terminalia arjuna (Roxb. ex DC.) Wight & Arnot as Potential Medicaments for Cardiovascular Disease: An In-Silico Approach.[Pubmed:36770716]
Molecules. 2023 Jan 20;28(3):1046.
Cardiovascular diseases (CVDs) are the leading cause of mortality worldwide. Terminalia arjuna (Roxb. ex DC.) Wight & Arnot of the Combretaceae family is one of the most frequently approved and utilized medicinal trees in the traditional medicinal system, which was used for the treatment of a variety of diseases, including cardiovascular disorders. The present study aims to identify phytochemicals from T. arjuna, that do not exhibit any toxicity and have significant cardioprotective activity using an in-silico technique. Four different cardiovascular proteins, namely human angiotensin receptor (PDB ID: 4YAY), P38 mitogen-activated protein kinase (MAPK, PDB ID: 4DLI), 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-Co A) reductase (PDB ID: 1HW9), and human C-reactive protein (PDB ID: 1B09), were used as target proteins to identify potential inhibitors using a virtual screening of the phytochemicals in T. arjuna revealed Casuarinin as a potential inhibitor of all selected target proteins with strong binding energy. Furthermore, MD simulations for a 100 ns time scale also revealed that most of the key protein contacts of all target proteins were retained throughout the simulation trajectories. Binding free energy calculations using the MM-GBSA approach also support a strong inhibitory effect of Casuarinin on target proteins. Casuarinin's effective binding to these proteins lays the groundwork for the development of broad-spectrum drugs as well as the understanding of the underlying mechanism against cardiovascular diseases through in vivo and clinical studies.
Association of Polygenic Variants Involved in Immunity and Inflammation with Duodenal Ulcer Risk and Their Interaction with Irregular Eating Habits.[Pubmed:36678166]
Nutrients. 2023 Jan 6;15(2):296.
Genetic and environmental factors are associated with developing and progressing duodenal ulcer (DU) risk. However, the exact nature of the disease pathophysiology and the single nucleotide polymorphism (SNP)-lifestyle interaction has yet to be determined. The purpose of the present study was to examine the SNPs linked to DU risk and their interaction with lifestyles and diets in a large hospital-based cohort of Asians. Based on an earlier diagnosis, the participants were divided into the DU (case; n = 1088) and non-DU (control, n = 56,713) groups. The SNP associated with DU risk were obtained from a genome-wide association study (GWAS), and those promoted genetic impact with SNP-SNP interactions were identified with generalized multifactor dimensionality reduction analysis. The interaction between polygenic risk score (PRS) calculated from the selected genetic variants and nutrient were examined. They were related to actin modification, immune response, and cell migration by modulating leucine-rich repeats (LRR) domain binding, Shaffer interferon regulatory factor 4 (IRF4) targets in myeloma vs. mature B lymphocyte, and Reactome runt-related transcription factor 3 (RUNX3). Among the selected SNPs, rs11230563 (R225W) showed missense mutation and low binding affinity with different food components in the autodock analysis. Glycyrrhizin, physalin B, janthitrem F, and Casuarinin lowered it in only wild CD6 protein but not in mutated CD6. Plastoquinone 8, solamargine, saponin D, and matesaponin 2 decreased energy binding affinity in mutated CD6 proteins. The PRS of the 5-SNP and 6-SNP models exhibited a positive association with DU risk (OR = 3.14). The PRS of the 5-SNP PRS model interacted with irregular eating habits and smoking status. In participants with irregular eating habits or smokers, DU incidence was much higher in the participants with high PRS than in those with low PRS. In conclusion, the genetic impact of DU risk was mainly in regulating immunity, inflammation, and actin modification. Adults who are genetically susceptible to DU need to eat regularly and to be non-smokers. The results could be applied to personalize nutrition.
Antiacne and Anti-Inflammatory Effects of Phenolic Compounds from Quercus acutissima Carruth. Leaves.[Pubmed:36624865]
Evid Based Complement Alternat Med. 2022 Dec 31;2022:9078475.
Quercus plants are widely distributed in Korea and have been used for their antiallergic and anti-inflammatory properties to treat dermatitis. The phenolic compounds of Quercus acutissima Carruth (QA) are estimated to have antioxidant and anti-inflammatory activities, based on the results of previous studies with Quercus mongilica, Quercus stenophylla, Quercus gilva Blame., and Quercus acuta Thunb. We yield QA extract and the isolated phenolic compounds (hyperoside (1), astragalin (2), kaempferol 3-O-(6''- galloyl)-beta-D-glucopyranoside (KGG) (3), quercetin 3-O-(6''-O-galloyl)-beta-D-glucopyranoside (QGG) (4), pedunculagin (5), and Casuarinin (6)) and were identified using NMR. Among them, KGG (3) and QGG (4) were isolated for the first time from QA. QA extract and the isolated phenolic compounds demonstrated antioxidative, anti-inflammatory, and antiacne activities in RAW 264.7 mouse macrophage cells in vitro. 3-6 demonstrated strong inhibitory activities in the DPPH scavenging and NO production assay and anti-inflammatory and antiacne activities through western blotting (NLRP3, IL-1beta, and 5alpha-reductase). The most outstanding activity in all experiments was Casuarinin (6). The study findings suggest potential therapeutic candidates for acne.
Structural determination and anticholinesterase assay of C-glycosidic ellagitannins from Lawsonia inermis leaves: A study supported by DFT calculations and molecular docking.[Pubmed:36423882]
Fitoterapia. 2023 Jan;164:105360.
An ellagitannin monomer, lythracin M (1), and a dimer, lythracin D (2), along with eight known monomers (3-10) were isolated from Lawsonia inermis (Lythraceae) leaves. Lythracin M (1) is a C-glycosidic ellagitannin with a flavogallonyl dilactone moiety that participates in the creation of a gamma-lactone ring with the anomeric carbon of the glucose core. Lythracin D (2) was determined as an atropisomer of the reported lythcarin D. These newly discovered structures (1 and 2) were determined by intensive spectroscopic experiments and by comparing DFT-calculated (1)H(1)H coupling, (1)H NMR chemical shifts, and ECD data with experimental values. The anti-acetylcholinesterase assay of the compounds 1-10 revealed that the C-1 ellagitannin epimers [Casuarinin (7; IC(50) = 34 +/- 2 nM) and stachyurin (8; IC(50) = 56 +/- 3 nM)], and the new dimer (2; IC(50) = 61 +/- 4 nM) possess enzyme inhibitory effects comparable to the reference drug (donepezil, IC(50) = 44 +/- 3 nM). Molecular docking of compounds 1-10 with AChE identified the free galloyl moiety as an important pharmacophore in the anticholinesterase activity of tannins.
Browning inhibition of seabuckthorn leaf extract on fresh-cut potato sticks during cold storage.[Pubmed:35489264]
Food Chem. 2022 Sep 30;389:133076.
Seabuckthorn extract is rich in bioactive compounds and well known for its health benefits. The study investigated the effect of seabuckthorn leaf extract on browning of fresh-cut potatoes. The results showed that seabuckthorn leaf extract significantly inhibited the browning of fresh-cut potatoes compared with seabuckthorn fruit extract. Catechin, hypericin, gallic acid, Casuarinin and isorhamnetin were main components in seabuckthorn leaf extract. Further research revealed that seabuckthorn leaf extract competitively inhibited polyphenol oxidase (PPO) with IC(50) value of 0.7 mg/mL. Molecular docking indicated that gallic acid stably bound to the active site of PPO, while isorhamnetin had low affinity on PPO. These results also demonstrated that seabuckthorn leaf extract inhibited browning of fresh-cut potatoes by reducing activities of peroxidase and phenylalanine ammonia-lyase, decreasing contents of phenolics and elevating antioxidant capacity. In addition, synergistic anti-browning effect was found with Casuarinin, isorhamnetin, gallic acid and pedunculagin.
Protective role of casuarinin from Melastoma malabathricum against a mouse model of 5-fluorouracil-induced intestinal mucositis: Impact on inflammation and gut microbiota dysbiosis.[Pubmed:35430483]
Phytomedicine. 2022 Jul;101:154092.
BACKGROUND: 5-FU-induced intestinal mucositis (FUIIM) is a common gastrointestinal side effect of chemotherapy, leading to gastric pain in clinical cancer patients. In a previous study, we demonstrated that neutrophil elastase (NE) inhibitors could alleviate FUIIM and manipulate the homeostasis of the gut microbiota. The root of Melastoma malabathricum, also called Ye-Mu-Dan, has been used as a traditional Chinese medicine for gastrointestinal disease. Water extract of the roots of M. malabathricum exhibits an inhibitory effect on NE, with an IC(50) value of 9.13 mug/ml. PURPOSE: In this study, we aimed to isolate an anti-NE compound from the root of M. malabathricum and to determine the protective effect of the bioactive component on a mouse model of FUIIM with respect to tissue damage, inflammation, intestinal barrier dysfunction, and gut microbiota dysbiosis. METHODS: A water extract of the roots of M. malabathricum was prepared and its major bioactive compound, was identified using bioactivity-guided fractionation. The effects of samples on the inhibition of NE activity were evaluated using enzymatic assays. To evaluate the effects of the bioactive compound in an FUIIM animal model, male C57BL/6 mice treated with or without Casuarinin (50 and 100 mg/kg/day, p.o.), and then received of 5-fluorouracil (50 mg/kg/day) intraperitoneally for 5 days to induce FUIIM. Histopathological staining was used to monitor the tissue damage, proliferation of intestinal crypts, and expression of tight junction proteins. The inflammation score was estimated by determining the levels of oxidative stress, neutrophil-related proteases, and proinflammatory cytokines in tissue and serum. The ecology of the gut microbiota was evaluated using 16S rRNA gene sequencing. RESULTS: Casuarinin had the most potent and selective effect against NE, with an IC(50) value of 2.79 +/- 0.07 muM. Casuarinin (100 mg/kg/day, p.o.) significantly improved 5-FU-induced body weight loss together with food intake reduction, and it also significantly reversed villus atrophy, restored the proliferative activity of the intestinal crypts, and suppressed inflammation and intestinal barrier dysfunction in the mouse model of FUIIM. Casuarinin also reversed 5-FU-induced gut microbiota dysbiosis, particularly the abundance of Actinobacteria, Candidatus Arthromitus, and Lactobacillus murinus, and the Firmicutes-to-Bacteroidetes ratio. CONCLUSION: This study firstly showed that Casuarinin isolated from the root part of M. malabathricum could be used as a NE inhibitor, whereas it could improve FUIIM by modulating inflammation, intestinal barrier dysfunction, and gut microbiota dysbiosis. In summary, exploring anti-NE natural product may provide a way to find candidate for improvement of FUIIM.
Lemon Myrtle (Backhousia citriodora) Extract and Its Active Compound, Casuarinin, Activate Skeletal Muscle Satellite Cells In Vitro and In Vivo.[Pubmed:35268053]
Nutrients. 2022 Mar 4;14(5):1078.
Sarcopenia is an age-related skeletal muscle atrophy. Exercise is effective in improving sarcopenia via two mechanisms: activation of skeletal muscle satellite cells (SCs) and stimulation of muscle protein synthesis. In contrast, most nutritional approaches for improving sarcopenia focus mainly on muscle protein synthesis, and little is known about SC activation. Here, we investigated the effect of lemon myrtle extract (LM) on SC activation both in vitro and in vivo. Primary SCs or myoblast cell lines were treated with LM or its derived compounds, and incorporation of 5-bromo-2'-deoxyuridine, an indicator of cell cycle progression, was detected by immunocytochemistry. We found that LM significantly activated SCs (p < 0.05), but not myoblasts. We also identified Casuarinin, an ellagitannin, as the active compound in LM involved in SC activation. The structure-activity relationship analysis showed that rather than the structure of each functional group of Casuarinin, its overall structure is crucial for SC activation. Furthermore, SC activation by LM and Casuarinin was associated with upregulation of interleukin-6 mRNA expression, which is essential for SC activation and proliferation. Finally, oral administration of LM or Casuarinin to rats showed significant activation of SCs in skeletal muscle (p < 0.05), suggesting that LM and Casuarinin may serve as novel nutritional interventions for improving sarcopenia through activating SCs.
Avens Root (Geum Urbanum L.) Extract Discovered by Target-Based Screening Exhibits Antidiabetic Activity in the Hen's Egg Test Model and Drosophila melanogaster.[Pubmed:34975489]
Front Pharmacol. 2021 Dec 15;12:794404.
Medicinal plant extracts are becoming increasingly important as an alternative for traditional drugs against diabetes mellitus (DM). For this reason, we initialized a target-based screening of 111 root extracts from an open access plant extract library (PECKISH) by ascertaining their in-vitro inhibitory efficacy on alpha-glucosidase. The two most active extracts Geum urbanum L. (roseroot) and Rhodiola rosea L. (avens root) were further tested for their antidiabetic activities in terms of their impact on different regulatory key points of glucose homeostasis. To this end, various enzyme- and cell culture-based in-vitro assays were employed including the determination of sodium-dependent glucose transporter 1 (SGLT1) activity in Caco-2 monolayers by Ussing chambers and of glucose transporter 4 (GLUT4) translocation in a GFP-reporter cell line. Subsequently, the antidiabetic potential of the root extracts were further evaluated in in-vivo models, namely hen's eggs test and the fruit fly Drosophila melanogaster. Avens root extract was found to be a more potent inhibitor of the enzymes alpha-glucosidase and dipeptidyl peptidase-4 (DPP4) than roseroot extract. Most importantly, only avens root extract exhibited antidiabetic activity in the two in-vivo models eliciting a reduced blood glucose level in the in-ovo model and a decline of the triglyceride level in a dietary starch-induced D. melanogaster obesity model. Analyses of the polyphenolic composition of the avens root extract by HPLC revealed a high content of ellagic acid and its derivatives as well as ellagitannins such as pedunculagin, stenophyllanin, stachyurin, Casuarinin and gemin A. In conclusion, avens root extract represents a promising medicinal plant that should be considered in further in-vivo studies on hyperglycemia in laboratory rodents and humans.
Repurposing of the herbal formulations: molecular docking and molecular dynamics simulation studies to validate the efficacy of phytocompounds against SARS-CoV-2 proteins.[Pubmed:33988079]
J Biomol Struct Dyn. 2022 Nov;40(18):8405-8419.
Herbal formulations mentioned in traditional medicinal texts were investigated for in silico effect against SARS-COV-2 proteins involved in various functions of a virus such as attachment, entry, replication, transcription, etc. To repurpose and validate polyherbal formulations, molecular docking was performed to study the interactions of more than 150 compounds from various formulations against the SARS-CoV-2 proteins. Molecular dynamics (MD) simulation was performed to evaluate the interaction of top scored ligands with the various receptor proteins. The docking results showed that Liquiritic acid, Liquorice acid, Terchebulin, Glabrolide, Casuarinin, Corilagin, Chebulagic acid, Neochebulinic acid, Daturataturin A, and Taraxerol were effective against SARS-COV-2 proteins with higher binding affinities with different proteins. Results of MD simulations validated the stability of ligands from potent formulations with various receptors of SARS-CoV-2. Binding free energy analysis suggested the favourable interactions of phytocompounds with the recpetors. Besides, in silico comparison of the various formulations determined that Pathyadi kwath, Sanjeevani vati, Yashtimadhu, Tribhuvan Keeratiras, and Septillin were more effective than Samshamni vati, AYUSH-64, and Trikatu. Polyherbal formulations having anti-COVID-19 potential can be used for the treatment with adequate monitoring. New formulations may also be developed for systematic trials based on ranking from these studies.Communicated by Ramaswamy H. Sarma.
Two new C-glycosidic ellagitannins and accompanying tannins from Lawsonia inermis leaves and their cytotoxic effects.[Pubmed:33984438]
Fitoterapia. 2021 Sep;153:104925.
Investigation on tannins having antitumor properties led to the isolation of two new C-glycosidic ellagitannins (1 and 2) along with seven known ellagitannins (3-9) and a related polyphenolic constituent (10) from Lawsonia inermis leaves. Our intensive HRESIMS, 1D and 2D NMR, and ECD spectroscopic studies of new tannins have shown that one (1) has a monomer structure of C-glycosidic tannin, and the other (2) has a dimeric structure of 2,3-O-hexahydroxydiphenoyl glucopyranose and a C-glycosidic tannin. Among the known compounds, one (3) is a C-glycosidic tannin that was isolated first of all from nature, five were C-glycosidic tannins, vescalagin (4), 1-O-methylvescalagin (5), castalagin (6), stachyurin (7), and Casuarinin (8), and one was an O-glycosidic ellagitannin, tellimagrandin II (9). The remaining phenolic constituent from the leaves was identified as valoneic acid dilactone (10). The ellagitannins 1, and 3-9 demonstrated noticeable cytotoxicity on human oral squamous cell carcinoma cell lines (HSC-2, HSC-4, and Ca9-22), and lower effects on human oral normal cells (HGF, HPC, and HPLF). Tellimagrandin II (9) had the highest tumor-specific cytotoxicity, and also cleaved poly (ADP-ribose) polymerase 1 in HSC-2 cells. These findings showed that L. inermis ellagitannins may be a candidate for the production of anti-oral cancer materials.
Phytochemical analysis and biological activities of ethanolic extract of Curcuma longa rhizome.[Pubmed:32965334]
Braz J Biol. 2021 Jul-Sep;81(3):737-740.
Curcuma longa is an important dietary plant which possess several pharmacological activities, including antioxidant, antimicrobial, anti-inflamatory, anticancer and anti clotting etc. The aim of the present study was to determine the phenolic profile of Curcuma longa and in vitro antioxidant and antidiabetic activities. In HPLC chromatogram of Curcuma longa rhizome extract 15 phenolic compounds were identified namely Digalloyl-hexoside, Caffeic acid hexoside, Curdione, Coumaric, Caffeic acid, Sinapic acid, Qurecetin-3-D-galactoside, Casuarinin, Bisdemethoxycurcumin, Curcuminol, Demethoxycurcumin, and Isorhamnetin, Valoneic acid bilactone, Curcumin, Curcumin-O-glucuronide respectively. The ethanolic extract displayed an IC50 value of 37.1+/-0.3 microg/ml against alpha glucosidase. The IC50 value of DPPH radical scavenging activity was 27.2 +/- 1.1 mug/mL. It is concluded that ethanolic extract of Curcuma long is rich source of curcumin and contain several important phenolics. The in vitro antioxidant and alpha glucosidase inhibitory effect of the plant justifies its popular use in traditional medicine.
Total Synthesis of Casuarinin.[Pubmed:32275157]
Org Lett. 2020 May 1;22(9):3392-3396.
This study involves the total synthesis of Casuarinin, a naturally occurring ellagitannin, in which an open-chain glucose is esterified with two (S)-hexahydroxydiphenoyl (HHDP) groups. One HHDP group incorporates a C-glycosidic bond between its benzene ring and the glucose moiety, which was constructed with complete stereoselectivity using a benzyl oxime group that opened the glucopyranose ring and acted as a scaffold for C-glycoside production. This total synthesis enables future structure-activity relationship studies of this compound.


