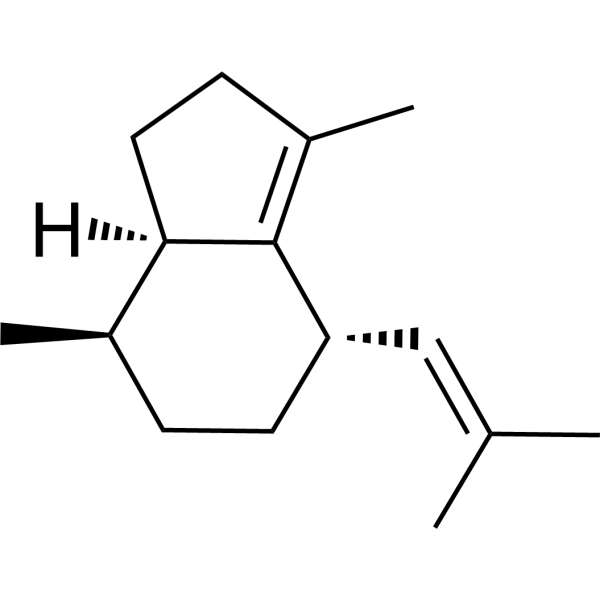
Valerena-4,7(11)-dieneCAS# 351222-66-7 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 351222-66-7 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C15H24 | M.Wt | 204.35 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Valerena-4,7(11)-diene Dilution Calculator

Valerena-4,7(11)-diene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8936 mL | 24.4678 mL | 48.9356 mL | 97.8713 mL | 122.3391 mL |
| 5 mM | 0.9787 mL | 4.8936 mL | 9.7871 mL | 19.5743 mL | 24.4678 mL |
| 10 mM | 0.4894 mL | 2.4468 mL | 4.8936 mL | 9.7871 mL | 12.2339 mL |
| 50 mM | 0.0979 mL | 0.4894 mL | 0.9787 mL | 1.9574 mL | 2.4468 mL |
| 100 mM | 0.0489 mL | 0.2447 mL | 0.4894 mL | 0.9787 mL | 1.2234 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Campneoside I
Catalog No.:BCX1844
CAS No.:95519-12-3
- Interiorin C
Catalog No.:BCX1843
CAS No.:133360-39-1
- Calarene
Catalog No.:BCX1842
CAS No.:17334-55-3
- β-Maaliene
Catalog No.:BCX1841
CAS No.:489-29-2
- Heteroclitin Ib
Catalog No.:BCX1840
CAS No.:1011762-96-1
- Casuarinin
Catalog No.:BCX1839
CAS No.:79786-01-9
- 23-O-β-D-Glucopyranosyl-20-isoveratramine
Catalog No.:BCX1838
CAS No.:148440-62-4
- Kadsurenin C
Catalog No.:BCX1837
CAS No.:145722-88-9
- (+)-Osbeckic acid
Catalog No.:BCX1836
CAS No.:112923-64-5
- Angeloylbinankadsurin A
Catalog No.:BCX1835
CAS No.:77165-80-1
- Schiarisanrin C
Catalog No.:BCX1834
CAS No.:130252-44-7
- Interiorin D
Catalog No.:BCX1833
CAS No.:133360-40-4
- (7R)-Isocampneoside I
Catalog No.:BCX1846
CAS No.:1119034-95-5
- Cynanoside J
Catalog No.:BCX1847
CAS No.:1800029-53-1
- Stauntoside R
Catalog No.:BCX1848
CAS No.:2172321-58-1
- Heteroclitin E
Catalog No.:BCX1849
CAS No.:140369-77-3
- Longipedunin A
Catalog No.:BCX1850
CAS No.:886441-72-1
- Heteroclitin G
Catalog No.:BCX1851
CAS No.:144027-74-7
- Schiarisanrin B
Catalog No.:BCX1852
CAS No.:130252-43-6
- Cynatratoside D
Catalog No.:BCX1853
CAS No.:97399-99-0
- Interiotherin D
Catalog No.:BCX1854
CAS No.:460090-66-8
- Stauntoside M
Catalog No.:BCX1855
CAS No.:1537167-62-6
- Kadsulignan C
Catalog No.:BCX1856
CAS No.:137637-49-1
- Kadsuphilin J
Catalog No.:BCX1857
CAS No.:1017242-94-2
Biosynthesis of valerenic acid by engineered Saccharomyces cerevisiae.[Pubmed:35643816]
Biotechnol Lett. 2022 Jul;44(7):857-865.
OBJECTIVE: To produce valerenic acid (VA) in Saccharomyces cerevisiae by engineering a heterologous synthetic pathway. RESULT: Valerena-4,7(11)-diene synthase (VDS) derived from Valeriana officinalis (valerian) was expressed in S. cerevisiae to generate Valerena-4,7(11)-diene as the precursor of VA. By overexpressing the key genes of the mevalonate pathway ERG8, ERG12 and ERG19, and integrating 4 copies of MBP (maltose-binding protein)-VDS-ERG20 gene expression caskets into the genome, the production of Valerena-4,7(11)-diene was improved to 75 mg/L. On this basis, the cytochrome P450 monooxygenase LsGAO2 derived from Lactuca sativa was expressed to oxidize Valerena-4,7(11)-diene to produce VA, and the most effective VA production strain was used for fermentation. The yield of VA reached 2.8 mg/L in the flask and 6.8 mg/L in a 5-L bioreactor fed glucose. CONCLUSIONS: An S. cerevisiae strain was constructed and optimized to produce VA, but the Valerena-4,7(11)-diene oxidation by LsGAO2 is still the rate-limiting step for VA synthesis that needs to be further optimized in future studies.
De novo synthesis of the sedative valerenic acid in Saccharomyces cerevisiae.[Pubmed:29545148]
Metab Eng. 2018 May;47:94-101.
Valeriana officinalis (Valerian) root extracts have been used by European and Asian cultures for millennia for their anxiolytic and sedative properties. However, the efficacy of these extracts suffers from variable yields and composition, making these extracts a prime candidate for microbial production. Recently, valerenic acid, a C15 sesquiterpenoid, was identified as the active compound that modulates the GABA(A) channel. Although the first committed step, Valerena-4,7(11)-diene synthase, has been identified and described, the complete valerenic acid biosynthetic pathway remains to be elucidated. Sequence homology and tissue-specific expression profiles of V. officinalis putative P450s led to the discovery of a V. officinalis Valerena-4,7(11)-diene oxidase, VoCYP71DJ1, which required coexpression with a V. officinalis alcohol dehydrogenase and aldehyde dehydrogenase to complete valerenic acid biosynthesis in yeast. Further, we demonstrated the stable integration of all pathway enzymes in yeast, resulting in the production of 140 mg/L of Valerena-4,7(11)-diene and 4 mg/L of valerenic acid in milliliter plates. These findings showcase Saccharomyces cerevisiae's potential as an expression platform for facilitating multiply-oxidized medicinal terpenoid pathway discovery, possibly paving the way for scale up and FDA approval of valerenic acid and other active compounds from plant-derived herbal medicines.
Chemical Composition of Nardostachys grandiflora Rhizome Oil from Nepal--A Contribution to the Chemotaxonomy and Bioactivity of Nardostachys.[Pubmed:26197553]
Nat Prod Commun. 2015 Jun;10(6):1067-70.
The essential oil from the dried rhizome of Nardostachys grandiflora, collected from Jaljale, Nepal, was obtained in 1.4% yield, and a total of 72 compounds were identified constituting 93.8% of the essential oil. The rhizome essential oil of N. grandiflora was mostly composed of calarene (9.4%), Valerena-4,7(11)-diene (7.1%), nardol A (6.0%), 1(10)-aristolen-9beta-ol (11.6%), jatamansone (7.9%), valeranal (5.6%), and cis-valerinic acid (5.7%). The chemical composition of N. grandiflora rhizome oil from Nepal is qualitatively very different than those from Indian, Chinese, and Pakistani Nardostachys essential oils. In this study we have evaluated the chemical composition and biological activities of N. grandiflora from Nepal. Additionally, 1(10)-aristolen-9beta-ol was isolated and the structure determined by NMR, and represents the first report of this compound from N. grandiflora. N. grandiflora rhizome oil showed in-vitro antimicrobial activity against Bacillus cereus, Escherichia coli, and Candida albicans (MIC = 156 mug/mL), as well as in-vitro cytotoxic activity on MCF-7 cells.
Inhalation administration of valerena-4,7(11)-diene from Nardostachys chinensis roots ameliorates restraint stress-induced changes in murine behavior and stress-related factors.[Pubmed:24882416]
Biol Pharm Bull. 2014;37(6):1050-5.
Dried Nardostachys chinensis roots contain sesquiterpenoids that are widely used as herbal tranquilizers. We previously identified the highly sedative sesquiterpenoid Valerena-4,7(11)-diene (VLD) from this plant. In the present study, we investigated stress reducing effects of VLD and the associated mechanisms of action. Application of 15-min restraint stresses induced excitatory behaviors in mice. Immobility times in the forced swim test and sleeping times in the pentobarbital sleep test were shortened in the stressed group by 47% and 43%, respectively, compared with the control group. Furthermore, restraint stress increased serum corticosterone levels by 75%, and cerebral serotonin (5-HT) and dopamine (DA) levels. Inhaled VLD (300 microg/cage) suppressed stress-induced excitatory behaviors and significantly reduced stress-induced blood corticosterone, cerebral 5-HT, and DA levels. These results suggest that VLD interacts with the hypothalamic-pituitary-adrenal axis and the sympathetic-adrenomedullary system. These interactions appear to involve GABAergic and D2 antagonist activities. Moreover, tests in anosmic and intravenously treated mice showed that the sedative effect of inhaled VLD was expressed via olfactory stimulation and pulmonary absorption. Although more studies are required to further elucidate the properties of this compound, our studies suggest that VLD may be an effective anti-stress aromatherapy for humans.
Enzymatic synthesis of valerena-4,7(11)-diene by a unique sesquiterpene synthase from the valerian plant (Valeriana officinalis).[Pubmed:22776156]
FEBS J. 2012 Sep;279(17):3136-46.
Valerian (Valeriana officinalis) is a popular medicinal plant in North America and Europe. Its root extract is commonly used as a mild sedative and anxiolytic. Among dozens of chemical constituents (e.g. alkaloids, iridoids, flavonoids, and terpenoids) found in valerian root, Valerena-4,7(11)-diene and valerenic acid (C15 sesquiterpenoid) have been suggested as the active ingredients responsible for the sedative effect. However, the biosynthesis of the Valerena-4,7(11)-diene hydrocarbon skeleton in valerian remains unknown to date. To identify the responsible terpene synthase, next-generation sequencing (Roche 454 pyrosequencing) was used to generate approximately 1 million transcript reads from valerian root. From the assembled transcripts, two sesquiterpene synthases were identified (VoTPS1 and VoTPS2), both of which showed predominant expression patterns in root. Transgenic yeast expressing VoTPS1 and VoTPS2 produced germacrene C/germacrene D and Valerena-4,7(11)-diene, respectively, as major terpene products. Purified VoTPS1 and VoTPS2 recombinant enzymes confirmed these activities in vitro, with competent kinetic properties (K(m) of approximately 10 mum and k(cat) of 0.01 s(-1) for both enzymes). The structure of the Valerena-4,7(11)-diene produced from the yeast expressing VoTPS2 was further substantiated by (13) C-NMR and GC-MS in comparison with the synthetic standard. This study demonstrates an integrative approach involving next-generation sequencing and metabolically engineered microbes to expand our knowledge of terpenoid diversity in medicinal plants.
Concise synthesis of Valerena-4,7(11)-diene, a highly active sedative, from valerenic acid.[Pubmed:20834146]
Biosci Biotechnol Biochem. 2010;74(9):1963-4.
A concise synthesis of Valerena-4,7(11)-diene with potent sedative activity was achieved in three steps involving, reduction of carboxylic acid, bromination of the resulting alcohol, and reduction of the bromide from valerenic acid in a 63% total yield. This synthetic method makes it possible to provide further materials for biological testing to realize comprehensive SAR studies.
Evaluation of volatile components from spikenard: valerena-4,7(11)-diene is a highly active sedative compound.[Pubmed:19452246]
J Nat Med. 2009 Oct;63(4):380-5.
Valerena-4,7(11)-diene and beta-maaliene were isolated from spikenard for the first time, and the effects of inhaling these compounds were investigated. Both compounds reduced the locomotor activity of mice dose-dependently, even at a low dose. Valerena-4,7(11)-diene had a particularly profound effect, with the strongest sedative activity observed at a dose of 0.06%. Caffeine-treated mice that showed an area under the curve (AUC) for locomotor activity that was double that of controls were calmed to normal levels by administration of Valerena-4,7(11)-diene. The continuous sleep time of pentobarbital-treated mice was prolonged by about 2.7 times with Valerena-4,7(11)-diene, an effect similar to that of chlorpromazine administered orally.
Pacifigorgianes and tamariscene as constituents of Frullania tamarisci and Valeriana officinalis.[Pubmed:11382249]
Phytochemistry. 2001 May;57(2):307-13.
The sesquiterpenoid constituents of the essential oils from the liverworts Frullania tamarisci, Frullania fragilifolia and of the angiosperm Valeriana officinalis were investigated. Tamariscene, a compound with a new sesquiterpene skeleton, Valerena-4,7(11)-diene and five new pacifigorgiadienes, namely pacifigorgia-1,10-diene, pacifigorgia-1(6),10-diene, pacifigorgia-1(9),10-diene, pacifigorgia-2,10-diene, and pacifigorgia-2(10),11-diene were isolated and identified. Structure elucidation was carried out by NMR spectroscopy and chemical correlations to establish absolute configurations. Compounds present in both the essential oils of the Frullania species and Valeriana officinalis were enantiomeric to each other. A plausible biogenetic relationship between the pacifigorgiane, valerenane and tamariscane skeletons is postulated. Pacifigorgia-6,11-diene, not yet detected in nature, was generated by dehydration and rearrangement of natural (-)-tamariscol.


