ChamaechromoneCAS# 93413-00-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 93413-00-4 | SDF | Download SDF |
| PubChem ID | 12084958 | Appearance | Powder |
| Formula | C30H22O10 | M.Wt | 542.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
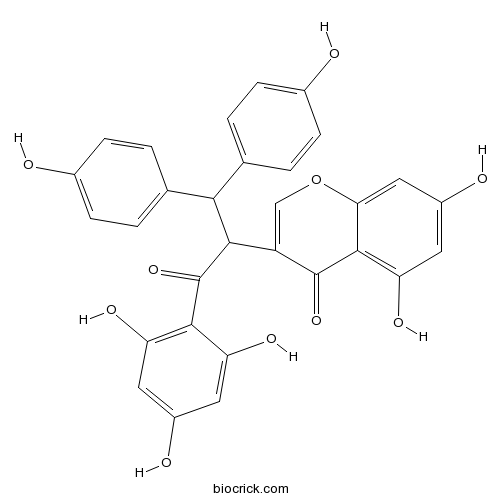
| Chemical Name | 3-[1,1-bis(4-hydroxyphenyl)-3-oxo-3-(2,4,6-trihydroxyphenyl)propan-2-yl]-5,7-dihydroxychromen-4-one | ||
| SMILES | C1=CC(=CC=C1C(C2=CC=C(C=C2)O)C(C3=COC4=CC(=CC(=C4C3=O)O)O)C(=O)C5=C(C=C(C=C5O)O)O)O | ||
| Standard InChIKey | KLKLIUIRQAMTAJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H22O10/c31-16-5-1-14(2-6-16)25(15-3-7-17(32)8-4-15)26(30(39)27-21(35)9-18(33)10-22(27)36)20-13-40-24-12-19(34)11-23(37)28(24)29(20)38/h1-13,25-26,31-37H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Chamaechromone has anti-HBV and insecticidal activity. 2. Chamaechromone can undergo extensive phase I and phase II metabolism in rat. 3. The hydroxylation of Chamaechromone is inhibited by α-naphthoflavone, and predominantly catalyzed by recombinant human cytochrome P450 1A2. |
| Targets | HBV | P450 (e.g. CYP17) |

Chamaechromone Dilution Calculator

Chamaechromone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8433 mL | 9.2166 mL | 18.4332 mL | 36.8664 mL | 46.0829 mL |
| 5 mM | 0.3687 mL | 1.8433 mL | 3.6866 mL | 7.3733 mL | 9.2166 mL |
| 10 mM | 0.1843 mL | 0.9217 mL | 1.8433 mL | 3.6866 mL | 4.6083 mL |
| 50 mM | 0.0369 mL | 0.1843 mL | 0.3687 mL | 0.7373 mL | 0.9217 mL |
| 100 mM | 0.0184 mL | 0.0922 mL | 0.1843 mL | 0.3687 mL | 0.4608 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-Benzothiazolol
Catalog No.:BCC8557
CAS No.:934-34-9
- 2-Aminobenzimidazole
Catalog No.:BCC8547
CAS No.:934-32-7
- 8beta-(2-Hydroxy-2-methyl-3-oxobutyryloxy)glucozaluzanin C
Catalog No.:BCN6682
CAS No.:93395-31-4
- 8-Epicrepiside E
Catalog No.:BCN7098
CAS No.:93395-30-3
- (S)-(-)-Atenolol
Catalog No.:BCC6633
CAS No.:93379-54-5
- (+)-Taddol
Catalog No.:BCC8378
CAS No.:93379-49-8
- 20-Hydroxy-3-oxo-28-lupanoic acid
Catalog No.:BCN4478
CAS No.:93372-87-3
- Monomethyl lithospermate
Catalog No.:BCN8124
CAS No.:933054-33-2
- Iodoacetyl-LC-Biotin
Catalog No.:BCC3584
CAS No.:93285-75-7
- (±)-PPCC oxalate
Catalog No.:BCC7796
CAS No.:932736-91-9
- CDDO-EA
Catalog No.:BCC5282
CAS No.:932730-51-3
- Boc-β-iodo-Ala-OMe
Catalog No.:BCC3052
CAS No.:93267-04-0
- Desvenlafaxine
Catalog No.:BCC5038
CAS No.:93413-62-8
- Venlafaxine
Catalog No.:BCC9190
CAS No.:93413-69-5
- A-966492
Catalog No.:BCC2211
CAS No.:934162-61-5
- GSK1014802(CNV1014802)
Catalog No.:BCC6454
CAS No.:934240-30-9
- HSP990 (NVP-HSP990)
Catalog No.:BCC5491
CAS No.:934343-74-5
- ENMD-2076
Catalog No.:BCC2186
CAS No.:934353-76-1
- Conantokin G
Catalog No.:BCC6120
CAS No.:93438-65-4
- Glucoliquiritin
Catalog No.:BCN6760
CAS No.:93446-18-5
- TAK-901
Catalog No.:BCC2180
CAS No.:934541-31-8
- 8-Deacetylyunaconitine
Catalog No.:BCN7609
CAS No.:93460-55-0
- Cobimetinib
Catalog No.:BCC1491
CAS No.:934660-93-2
- Cobimetinib (R-enantiomer)
Catalog No.:BCC1493
CAS No.:934660-94-3
Metabolism of chamaechromone in vitro with human liver microsomes and recombinant human drug-metabolizing enzymes.[Pubmed:24687737]
Planta Med. 2014 Apr;80(6):493-7.
Chamaechromone is a major component in the dried roots of Stellera chamaejasme with antihepatitis B virus and insecticidal activity. In this study, metabolic profiles of Chamaechromone were investigated in human liver microsomes. One monohydroxide and two monoglucuronides of Chamaechromone were identified. The enzyme kinetics for both hydroxylation and glucuronidation were fitted to the Michaelis-Menten equation. The hydroxylation of Chamaechromone was inhibited by alpha-naphthoflavone, and predominantly catalyzed by recombinant human cytochrome P450 1A2, whereas the glucuronidation was inhibited by quercetin, 1-naphthol, and fluconazole, and mainly catalyzed by recombinant human UDP-glucuronosyltransferase 1A3, 1A7, 1A9, and 2B7.
Metabolites characterization of chamaechromone in vivo and in vitro by using ultra-performance liquid chromatography/Xevo G2 quadrupole time-of-flight tandem mass spectrometry.[Pubmed:24189033]
J Ethnopharmacol. 2014;151(1):242-52.
ETHNOPHARMACOLOGICAL RELEVANCE: Stellera chamaejasme L. (Thymelaeaceae) was a toxic perennial herb and widely used as pesticide and dermatological agents in China. Chamaechromone was a major component in the dried roots of Stellera chamaejasme with anti-HBV and insecticidal activity. Analysis of metabolic profile in vivo and in vitro plays a pivotal role to unravel how TCM works. And the metabolites of Chamaechromone might influence the effects and toxicity of Stellera chamaejasme. Moreover, the metabolic routes of Chamaechromone provide an important basis for toxicological safety evaluation. Until now, little is known about the metabolism of Chamaechromone. The current study was designed to characterize the whole metabolic pathways of Chamaechromone in vitro and in vivo. MATERIALS AND METHODS: Twenty-four rats were randomly divided into four groups, including two oral administration groups (100mgkg(-1)), one intravenous injection group (5 mgkg(-1)), and one control group. The metabolites in rat urine and feces and bile were identified by UPLC/Q-TOF MS analysis and beta-glucuronidase hydrolysis. Moreover, the possible metabolic mechanism was further confirmed by Phase I and Phase II metabolism and catechol-O-methyltransferase methylation in rat liver S9 fraction and degradation in rat intestinal bacteria. RESULTS: A total of 24 metabolites from Chamaechromone were detected and identified in vivo and in vitro, 20 of which were novel. And the major metabolic processes were hydroxylation, methylation, glucuronation, acetylation, dehydroxylation and degradation. CONCLUSIONS: The present study revealed the whole metabolic pathways of Chamaechromone in rat through both in vitro and in vivo experiments for the first time. And Chamaechromone could undergo extensive phase I and phase II metabolism in rat. These findings would provide an important basis for the further study and clinical application of Chamaechromone. In addition, the results of this work have showed the feasibility of the UPLC/Q-TOF-MS approach for rapid and reliable characterization of metabolites.


