(S)-(-)-AtenololCAS# 93379-54-5 |
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

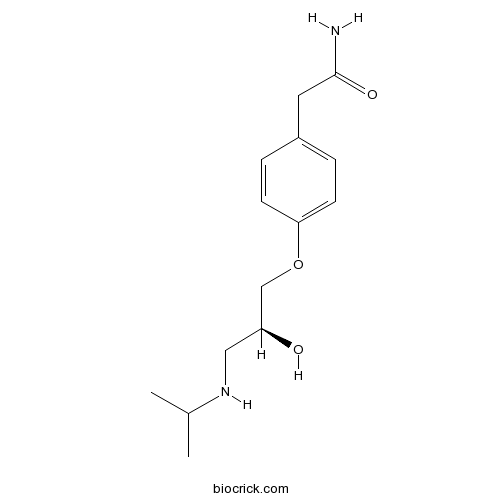
| Cas No. | 93379-54-5 | SDF | Download SDF |
| PubChem ID | 175540 | Appearance | Powder |
| Formula | C14H22N2O3 | M.Wt | 266.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in water | ||
| Chemical Name | 2-[4-[(2S)-2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl]acetamide | ||
| SMILES | CC(C)NCC(COC1=CC=C(C=C1)CC(=O)N)O | ||
| Standard InChIKey | METKIMKYRPQLGS-LBPRGKRZSA-N | ||
| Standard InChI | InChI=1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18)/t12-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | The active enantiomer of (RS)-atenolol, a cardioselective β-adrenergic blocker. |

(S)-(-)-Atenolol Dilution Calculator

(S)-(-)-Atenolol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7546 mL | 18.773 mL | 37.546 mL | 75.092 mL | 93.865 mL |
| 5 mM | 0.7509 mL | 3.7546 mL | 7.5092 mL | 15.0184 mL | 18.773 mL |
| 10 mM | 0.3755 mL | 1.8773 mL | 3.7546 mL | 7.5092 mL | 9.3865 mL |
| 50 mM | 0.0751 mL | 0.3755 mL | 0.7509 mL | 1.5018 mL | 1.8773 mL |
| 100 mM | 0.0375 mL | 0.1877 mL | 0.3755 mL | 0.7509 mL | 0.9386 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Taddol
Catalog No.:BCC8378
CAS No.:93379-49-8
- 20-Hydroxy-3-oxo-28-lupanoic acid
Catalog No.:BCN4478
CAS No.:93372-87-3
- Monomethyl lithospermate
Catalog No.:BCN8124
CAS No.:933054-33-2
- Iodoacetyl-LC-Biotin
Catalog No.:BCC3584
CAS No.:93285-75-7
- (±)-PPCC oxalate
Catalog No.:BCC7796
CAS No.:932736-91-9
- CDDO-EA
Catalog No.:BCC5282
CAS No.:932730-51-3
- Boc-β-iodo-Ala-OMe
Catalog No.:BCC3052
CAS No.:93267-04-0
- Cistanoside A
Catalog No.:BCN2668
CAS No.:93236-42-1
- 5-Aminouracil
Catalog No.:BCC8737
CAS No.:932-52-5
- 2-Hydroxybenzylamine
Catalog No.:BCN1803
CAS No.:932-30-9
- PPQ-102
Catalog No.:BCC5248
CAS No.:931706-15-9
- IOX2(Glycine)
Catalog No.:BCC2229
CAS No.:931398-72-0
- 8-Epicrepiside E
Catalog No.:BCN7098
CAS No.:93395-30-3
- 8beta-(2-Hydroxy-2-methyl-3-oxobutyryloxy)glucozaluzanin C
Catalog No.:BCN6682
CAS No.:93395-31-4
- 2-Aminobenzimidazole
Catalog No.:BCC8547
CAS No.:934-32-7
- 2-Benzothiazolol
Catalog No.:BCC8557
CAS No.:934-34-9
- Chamaechromone
Catalog No.:BCN3718
CAS No.:93413-00-4
- Desvenlafaxine
Catalog No.:BCC5038
CAS No.:93413-62-8
- Venlafaxine
Catalog No.:BCC9190
CAS No.:93413-69-5
- A-966492
Catalog No.:BCC2211
CAS No.:934162-61-5
- GSK1014802(CNV1014802)
Catalog No.:BCC6454
CAS No.:934240-30-9
- HSP990 (NVP-HSP990)
Catalog No.:BCC5491
CAS No.:934343-74-5
- ENMD-2076
Catalog No.:BCC2186
CAS No.:934353-76-1
- Conantokin G
Catalog No.:BCC6120
CAS No.:93438-65-4
Synthesis of a nano-sized chiral imprinted polymer and its use as an (S)-atenolol carrier in the bulk liquid membrane.[Pubmed:24771633]
J Sep Sci. 2014 Jul;37(14):1887-95.
In this work, nanosized chiral imprinted polymers containing (S)-atenolol ((S)-ATN) selective sites were synthesized by using suspension polymerization in silicon oil. (S)-ATN, methacrylic acid, and ethylene glycol dimethacrylate were used as enantiomerically pure template, functional monomer, and cross-linker, respectively. The prepared chiral imprinted polymers were used as the carrier elements in a bulk liquid membrane (BLM). (S)-ATN transport capability of the chiral imprinted polymers was compared with that of the nonimprinted polymer. It was shown that chiral imprinted polymers could transport (S)-ATN through the BLM more effectively than (R)-ATN, whereas no difference in the facilitated transport was observed between (R)-ATN and (S)-ATN when using nonimprinted polymer particles as the carrier element in the BLM. A kinetic model was proposed for the transportation of (S)-ATN through the chiral imprinted polymers based BLM. It was found that the extraction of ATN from the source to the membrane controls the chiral separation process. It was also found that the pH of source and receiving phases as well as the racemic ATN concentration in source phase had very crucial effect on the chiral separation efficiency.
Design, Development and Optimization of S (-) Atenolol Floating Sustained Release Matrix Tablets Using Surface Response Methodology.[Pubmed:26798171]
Indian J Pharm Sci. 2015 Sep-Oct;77(5):563-72.
The objective of this present investigation was to develop and formulate floating sustained release matrix tablets of s (-) atenolol, by using different polymer combinations and filler, to optimize by using surface response methodology for different drug release variables and to evaluate the drug release pattern of the optimized product. Floating sustained release matrix tablets of various combinations were prepared with cellulose-based polymers: Hydroxypropyl methylcellulose, sodium bicarbonate as a gas generating agent, polyvinyl pyrrolidone as a binder and lactose monohydrate as filler. The 3(2) full factorial design was employed to investigate the effect of formulation variables on different properties of tablets applicable to floating lag time, buoyancy time, % drug release in 1 and 6 h (D1 h,D6 h) and time required to 90% drug release (t90%). Significance of result was analyzed using analysis of non variance and P < 0.05 was considered statistically significant. S (-) atenolol floating sustained release matrix tablets followed the Higuchi drug release kinetics that indicates the release of drug follows anomalous (non-Fickian) diffusion mechanism. The developed floating sustained release matrix tablet of improved efficacy can perform therapeutically better than a conventional tablet.
Effect of aged garlic extract and s-allyl cysteine and their interaction with atenolol during isoproterenol induced myocardial toxicity in rats.[Pubmed:24550592]
Indian J Pharmacol. 2014 Jan-Feb;46(1):94-9.
OBJECTIVES: The study evaluates the cardioprotective effect of aged garlic extract (AGE) and its constituent; S-allylcysteine (SAC) and their interaction with atenolol during isoproterenol induced cardiac toxicity in rats. MATERIALS AND METHODS: Rats were administered AGE at two different doses of 2 ml/kg or 5 ml/kg orally whereas SAC was administered either at a dose 13.1 mg/kg or 32.76 mg/kg. The AGE or SAC was given alone or in combination with atenolol (6 mg/kg, p.o), every alternate day for three weeks. At the end of treatment, two doses of isoproterenol (150 mg/kg, s.c) were administered to rats. The electrocardiogram (ECG) was recorded followed by withdrawal of blood to estimate serum lactate dehydrogenase (LDH) and creatinine kinase-MB (CK-MB) activities. The activities of LDH, CK-MB as well as superoxide dismutase (SOD), catalase and thiobarbituric acid reactive substances (TBARS) were also determined in the heart tissue homogenate (HTH). RESULTS: The isoproterenol induced ECG changes were restored to normal in all treated groups. The AGE and SAC administration caused a decrease in serum LDH and CK-MB activities and an elevation of LDH and CK-MB activities in HTH. Atenolol alone or in combination with AGE and S-allylcsyteine demonstrated similar changes in biomarker activities. CONCLUSION: AGE showed dose-dependent cardioprotection. However, concurrent administration of SAC with atenolol (6 mg/kg, p.o) combated more effectively the myocardial dysfunction during isoproterenol induced cardiotoxicity in rats.
Stereoselective features of (R)- and (S)-atenolol: clinical pharmacological, pharmacokinetic, and radioligand binding studies.[Pubmed:8383518]
Chirality. 1993;5(1):15-9.
In a randomized, double-blind, cross-over study in 12 healthy volunteers, the effects of single oral doses of 100 mg rac-atenolol were compared during exercise to those of equal amounts of the optically pure enantiomers, i.e., 50 mg (R)- and 50 mg (S)-atenolol. The mean rate pressure product decreased with rac-atenolol (-37%; P < 0.01) and half-dosed (S)-atenolol (-35%; P < 0.01) to the same extent, whereas (R)-atenolol caused no effect. Radioligand binding studies in beta-adrenergic receptors of the guinea pig heart yielded a eudismic ratio of 46 for (S)- to (R)-atenolol. The mean AUCs, maximal plasma concentrations, and plasma half-lives of the enantiomers were similar regardless of whether they were administered as optically pure enantiomers or as racemic mixture. On the other hand, the AUC of (R)-atenolol was 1.08-fold greater (P < 0.01) than that of the (S)-enantiomer. The reason for this finding remains unclear. We conclude that only (S)-atenolol, but not (R)-atenolol, contributes to the beta-blocking effect of currently used rac-atenolol since the same effect can be elicited with the (S)-enantiomer alone.


