ENMD-2076Selective Aurora A/Flt3 inhibitor CAS# 934353-76-1 |
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
- CCT137690
Catalog No.:BCC2188
CAS No.:1095382-05-0
- CYC116
Catalog No.:BCC2181
CAS No.:693228-63-6
- MLN8054
Catalog No.:BCC2170
CAS No.:869363-13-3
- TAK-901
Catalog No.:BCC2180
CAS No.:934541-31-8
- PF-03814735
Catalog No.:BCC2184
CAS No.:942487-16-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 934353-76-1 | SDF | Download SDF |
| PubChem ID | 16041424 | Appearance | Powder |
| Formula | C21H25N7 | M.Wt | 375.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 31 mg/mL (82.56 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
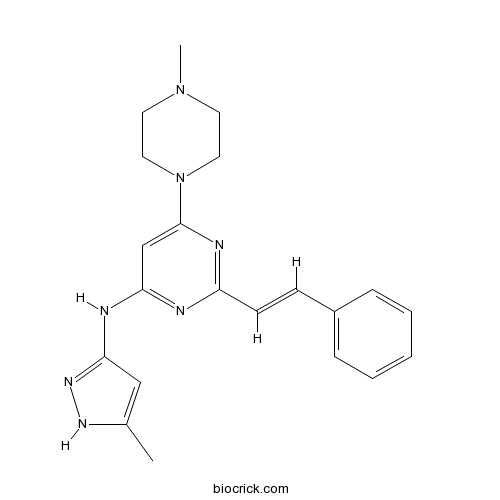
| Chemical Name | 6-(4-methylpiperazin-1-yl)-N-(5-methyl-1H-pyrazol-3-yl)-2-[(E)-2-phenylethenyl]pyrimidin-4-amine | ||
| SMILES | CC1=CC(=NN1)NC2=NC(=NC(=C2)N3CCN(CC3)C)C=CC4=CC=CC=C4 | ||
| Standard InChIKey | BLQYVHBZHAISJM-CMDGGOBGSA-N | ||
| Standard InChI | InChI=1S/C21H25N7/c1-16-14-20(26-25-16)23-19-15-21(28-12-10-27(2)11-13-28)24-18(22-19)9-8-17-6-4-3-5-7-17/h3-9,14-15H,10-13H2,1-2H3,(H2,22,23,24,25,26)/b9-8+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | ENMD-2076 is a selective inhibitor of Aurora A and Flt3 with IC50 values of 14 nM and 1.86 nM, respectively. | |||||
| Targets | Aurora A | Flt3 | ||||
| IC50 | 14 nM | 1.86 nM | ||||

ENMD-2076 Dilution Calculator

ENMD-2076 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6633 mL | 13.3166 mL | 26.6333 mL | 53.2666 mL | 66.5832 mL |
| 5 mM | 0.5327 mL | 2.6633 mL | 5.3267 mL | 10.6533 mL | 13.3166 mL |
| 10 mM | 0.2663 mL | 1.3317 mL | 2.6633 mL | 5.3267 mL | 6.6583 mL |
| 50 mM | 0.0533 mL | 0.2663 mL | 0.5327 mL | 1.0653 mL | 1.3317 mL |
| 100 mM | 0.0266 mL | 0.1332 mL | 0.2663 mL | 0.5327 mL | 0.6658 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ENMD-2076 is a selective activator of Aurora A and VEGFR with IC50 value of 14 and 1.86 nM[1].
Aurora kinases contains aurora A, aurora B and aurora C. They are serine/threonine kinases witch play an important role in cell proliferation. Aurora kinases are essential for cellular division by controlling segregation of chromatid. Aurora A is required for correct function of the centrosomes during the prophase of mitosis [1]. VEGFR (VEGF receptors) are receptors for VEGF (vascular endothelial growth factor). There are VEGFR 1, 2 and 3 which three main subtypes of VEGFR. The VEGFRs are tyrosine kinase receptors which are activited by binding to VEGFs then mediate the cellular responses to VEGF including the formation of the circulatory system (vasculogenesis) and the growth of blood vessels(angiogenesis) [1].
ENMD-2076 indicates activities of RET, FLT3, FLT4/VEGFR3, SRC, CSF1R/FMS, NTRK1, FGFR1/2, VEGFR2/KDR, LCK, and PDGFRα which all are kinases involved in angiogenes with IC50 from 1.86-120 nM. ENMD-2076 can inhibit the growth of hematopoietic cancer cell lines and lots of solid tumor with IC50 values from 25 to 700 nM in vitro[1]. ENMD-2076 significantly caused cytotoxicity in multiple myeloma (MM) cell lines and primary cells. ENMD-2076 also inhibited the phosphoinositide 3-kinase (PI3K)/AKT pathway. ENMD-2076 also induced cell cycle arrest in G2/M phase by inhibiting the activities of aurora kinases containing A and B class.[2]
ENMD-2076 regresses formed vessels and prevents new blood vessels formation in tumorxenograft models. [1] ENMD-2076 resulted in inhibition of tumour growth with oral treatment a dose-dependent manner with 50, 100, 200 mg/kg per day with human plasmacytoma xenografts[2].
References:
[1]. Fletcher GC, Brokx RD, Denny TA, Hembrough TA, Plum SM, Fogler WE, Sidor CF, Bray MR: ENMD-2076 is an orally active kinase inhibitor with antiangiogenic and antiproliferative mechanisms of action. Mol Cancer Ther 2011, 10(1):126-137.
[2]. Wang X, Sinn AL, Pollok K, Sandusky G, Zhang S, Chen L, Liang J, Crean CD, Suvannasankha A, Abonour R et al: Preclinical activity of a novel multiple tyrosine kinase and aurora kinase inhibitor, ENMD-2076, against multiple myeloma. Br J Haematol 2010, 150(3):313-325.
- HSP990 (NVP-HSP990)
Catalog No.:BCC5491
CAS No.:934343-74-5
- GSK1014802(CNV1014802)
Catalog No.:BCC6454
CAS No.:934240-30-9
- A-966492
Catalog No.:BCC2211
CAS No.:934162-61-5
- Venlafaxine
Catalog No.:BCC9190
CAS No.:93413-69-5
- Desvenlafaxine
Catalog No.:BCC5038
CAS No.:93413-62-8
- Chamaechromone
Catalog No.:BCN3718
CAS No.:93413-00-4
- 2-Benzothiazolol
Catalog No.:BCC8557
CAS No.:934-34-9
- 2-Aminobenzimidazole
Catalog No.:BCC8547
CAS No.:934-32-7
- 8beta-(2-Hydroxy-2-methyl-3-oxobutyryloxy)glucozaluzanin C
Catalog No.:BCN6682
CAS No.:93395-31-4
- 8-Epicrepiside E
Catalog No.:BCN7098
CAS No.:93395-30-3
- (S)-(-)-Atenolol
Catalog No.:BCC6633
CAS No.:93379-54-5
- (+)-Taddol
Catalog No.:BCC8378
CAS No.:93379-49-8
- Conantokin G
Catalog No.:BCC6120
CAS No.:93438-65-4
- Glucoliquiritin
Catalog No.:BCN6760
CAS No.:93446-18-5
- TAK-901
Catalog No.:BCC2180
CAS No.:934541-31-8
- 8-Deacetylyunaconitine
Catalog No.:BCN7609
CAS No.:93460-55-0
- Cobimetinib
Catalog No.:BCC1491
CAS No.:934660-93-2
- Cobimetinib (R-enantiomer)
Catalog No.:BCC1493
CAS No.:934660-94-3
- Cobimetinib (racemate)
Catalog No.:BCC1492
CAS No.:934662-91-6
- Karavilagenin D
Catalog No.:BCN4480
CAS No.:934739-29-4
- Glimepiride
Catalog No.:BCC2109
CAS No.:93479-97-1
- 2-Iodomelatonin
Catalog No.:BCC6772
CAS No.:93515-00-5
- (4->2)-Abeo-16-hydroxycleroda-2,13-dien-15,16-olide-3-al
Catalog No.:BCN7498
CAS No.:935293-70-2
- TH-237A
Catalog No.:BCC5378
CAS No.:935467-97-3
[Killing effect of aurora kinase inhibitor ENMD-2076 on acute myelogenous leukemia cells].[Pubmed:23086638]
Zhejiang Da Xue Xue Bao Yi Xue Ban. 2012 Sep;41(5):479-84.
OBJECTIVE: To investigate the effect of aurora kinase inhibitor ENMD-2076 on human acute myelogenous leukemia (AML) cell lines. METHODS: AML THP-1 and Kasumi-1 cells were treated with ENMD-2076 for 24 h and 48 h,respectively. Cell growth was measured by MTT assay. Apoptosis was determined using Hoechst staining apoptosis detection kit. Activation of Caspase pathway and expression of apoptosis regulator proteins were detected by Western blot. RESULTS: ENMD-2076 significantly induced growth arrest and apoptosis in THP-1 and Kasumi-1 cells. Enhanced apoptosis was observed in ENMD-2076 group evidenced by strong activation of Caspase-9,Caspase-3 and PARP. Furthermore,the ENMD-2076 treatment resulted in down-regulation of anti-apoptotic protein Mcl-1 expression. Also,up-regulated expression of pro-apoptotic protein Bak,Bad and Bax was detected after ENMD-2076 treatment. CONCLUSION: ENMD-2076 can kill effectively AML cells by inhibiting cell growth and inducing apoptosis,which is associated with activation of Caspase pathway and regulation of pro-apoptotic and anti-apoptotic proteins.
A phase I trial of the aurora kinase inhibitor, ENMD-2076, in patients with relapsed or refractory acute myeloid leukemia or chronic myelomonocytic leukemia.[Pubmed:27406088]
Invest New Drugs. 2016 Oct;34(5):614-24.
ENMD-2076 is a novel, orally-active molecule that inhibits Aurora A kinase, as well as c-Kit, FLT3 and VEGFR2. A phase I study was conducted to determine the maximum tolerated dose (MTD), recommended phase 2 dose (RP2D) and toxicities of ENMD-2076 in patients with acute myeloid leukemia (AML) and chronic myelomonocytic leukemia (CMML). Patients received escalating doses of ENMD-2076 administered orally daily [225 mg (n = 7), 375 mg (n = 6), 325 mg (n = 9), or 275 mg (n = 5)]. Twenty-seven patients were treated (26 AML; 1 CMML-2). The most common non-hematological toxicities of any grade, regardless of association with drug, were fatigue, diarrhea, dysphonia, dyspnea, hypertension, constipation, and abdominal pain. Dose-limiting toxicities (DLTs) consisted of grade 3 fatigue, grade 3 typhilitis, grade 3 syncope and grade 3 QTc prolongation). Of the 16 evaluable patients, one patient achieved a complete remission with incomplete count recovery (CRi), three experienced a morphologic leukemia-free state (MLFS) with a major hematologic improvement in platelets (HI-P), and 5 other patients had a reduction in marrow blast percentage (i.e. 11-65 %). The RP2D in this patient population is 225 mg orally once daily.
The radiosensitizing effect of the aurora kinase inhibitors, ENMD-2076, on canine mast cell tumours in vitro.[Pubmed:23763774]
Vet Comp Oncol. 2016 Mar;14(1):13-27.
ENMD-2076 is an aurora kinase inhibitor that also has multi-target tyrosine kinase inhibitor properties. In this study, the mRNA and the protein expression of aurora-A and aurora-B were evaluated in three canine mast cell tumour cell lines. Dose-dependent cytotoxicity was seen in the cells treated, and it affected the cell cycle with cells in the G2/M phase being selectively killed. The cells were also evaluated for radiosensitivity with/without ENMD-2076, and radiosensitization was seen after 3 Gy and 6 Gy exposures with ENMD-2076 for 48 h. Protein expression of caspase-3 was gradually increased, and the expression intensity was highest at 24 h post irradiation in cells without ENMD-2076 treatment, which indicates that radiation exposure with ENMD-2076-induced cell death faster than radiation treatment alone. Our study results suggest the potential usefulness of treating canine mast cell tumours with aurora kinase inhibitors alone or in conjunction with radiation therapy.
Predictive biomarkers of sensitivity to the aurora and angiogenic kinase inhibitor ENMD-2076 in preclinical breast cancer models.[Pubmed:23136197]
Clin Cancer Res. 2013 Jan 1;19(1):291-303.
PURPOSE: The Aurora kinases are a family of conserved serine-threonine kinases with key roles in mitotic cell division. As with other promising anticancer targets, patient selection strategies to identify a responsive subtype will likely be required for successful clinical development of Aurora kinase inhibitors. The purpose of this study was to evaluate the antitumor activity of the Aurora and angiogenic kinase inhibitor ENMD-2076 against preclinical models of breast cancer with identification of candidate predictive biomarkers. EXPERIMENTAL DESIGN: Twenty-nine breast cancer cell lines were exposed to ENMD-2076 and the effects on proliferation, apoptosis, and cell-cycle distribution were evaluated. In vitro activity was confirmed in MDA-MB-468 and MDA-MB-231 triple-negative breast cancer xenografts. Systematic gene expression analysis was used to identify up- and downregulated pathways in the sensitive and resistant cell lines, including within the triple-negative breast cancer subset. RESULTS: ENMD-2076 showed antiproliferative activity against breast cancer cell lines, with more robust activity against cell lines lacking estrogen receptor expression and those without increased HER2 expression. Within the triple-negative breast cancer subset, cell lines with a p53 mutation and increased p53 expression were more sensitive to the cytotoxic and proapoptotic effects of ENMD-2076 exposure than cell lines with decreased p53 expression. CONCLUSIONS: ENMD-2076 exhibited robust anticancer activity against models of triple-negative breast cancer and the candidate predictive biomarkers identified in this study may be useful in selecting patients for Aurora kinase inhibitors in the future.


