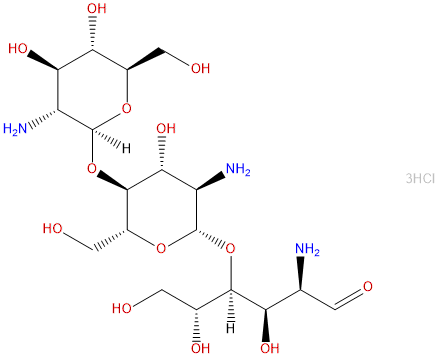
Chitotriose TrihydrochlorideCAS# 117436-78-9 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 117436-78-9 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C18H38Cl3N3O13 | M.Wt | 610.86 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Chitotriose Trihydrochloride Dilution Calculator

Chitotriose Trihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.637 mL | 8.1852 mL | 16.3704 mL | 32.7407 mL | 40.9259 mL |
| 5 mM | 0.3274 mL | 1.637 mL | 3.2741 mL | 6.5481 mL | 8.1852 mL |
| 10 mM | 0.1637 mL | 0.8185 mL | 1.637 mL | 3.2741 mL | 4.0926 mL |
| 50 mM | 0.0327 mL | 0.1637 mL | 0.3274 mL | 0.6548 mL | 0.8185 mL |
| 100 mM | 0.0164 mL | 0.0819 mL | 0.1637 mL | 0.3274 mL | 0.4093 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Chitobiose Octaacetate
Catalog No.:BCX1420
CAS No.:41670-99-9
- Chitotriose Undecaacetate
Catalog No.:BCX1419
CAS No.:53942-45-3
- Chitotetraose tetradecaacetate
Catalog No.:BCX1418
CAS No.:53942-46-4
- Chitopentaose Pentadecaacetate
Catalog No.:BCX1417
CAS No.:117399-52-7
- N,N'-Diacetylchitobiose
Catalog No.:BCX1416
CAS No.:35061-50-8
- N,N',N''-Triacetylchitotriose
Catalog No.:BCX1415
CAS No.:38864-21-0
- N,N',N'',N'''-Tetraacetylchitotetraose
Catalog No.:BCX1414
CAS No.:2706-65-2
- N,N',N'',N''',N''''-Pentaacetyl chitopentaose
Catalog No.:BCX1413
CAS No.:36467-68-2
- N,N',N'',N''',N'''',N'''''-Hexaacetyl chitohexaose
Catalog No.:BCX1412
CAS No.:38854-46-5
- Chitobiose Dihydrochloride
Catalog No.:BCX1411
CAS No.:115350-24-8
- Chitotetraose Tetrahydrochloride
Catalog No.:BCX1410
CAS No.:117399-50-5
- Chitopentaose Pentahydrochloride
Catalog No.:BCX1409
CAS No.:117467-64-8
- Chitooctaose Octahydrochloride
Catalog No.:BCX1422
CAS No.:127171-90-8
- Chitoheptaose Heptahydrochloride
Catalog No.:BCX1423
CAS No.:127171-89-5
- L-octaguluronic acid octasodium salt
Catalog No.:BCX1424
CAS No.:862694-88-0
- L-heptaguluronic acid heptasodium salt
Catalog No.:BCX1425
CAS No.:862694-87-9
- L-hexaguluronic acid hexasodium salt
Catalog No.:BCX1426
CAS No.:183668-74-8
- L-pentaguluronic acid pentasodium salt
Catalog No.:BCX1427
CAS No.:183668-72-6
- L-tetraguluronic acid tetrasodium salt
Catalog No.:BCX1428
CAS No.:149511-37-5
- L-triguluronic acid trisodium salt
Catalog No.:BCX1429
CAS No.:66754-14-1
- L-diguluronic acid disodium salt
Catalog No.:BCX1430
CAS No.:34044-54-7
- D-nonamannuronic acid nonasodium salt
Catalog No.:BCX1431
CAS No.:862694-99-3
- D-octamannuronic acid octasodium salt
Catalog No.:BCX1432
CAS No.:862694-98-2
- D-heptamannuronic acid heptasodium salt
Catalog No.:BCX1433
CAS No.:862694-97-1
A novel method for chemo-enzymatic synthesis of elicitor-active chitosan oligomers and partially N-deacetylated chitin oligomers using N-acylated chitotrioses as substrates in a lysozyme-catalyzed transglycosylation reaction system.[Pubmed:8593620]
Carbohydr Res. 1995 Dec 27;279:151-60.
N,N',N"-Tri(monochloro)acetylchitotriose prepared by N-monochloroacetylation of Chitotriose Trihydrochloride was successfully polymerized into higher-molecular-weight oligomers by a lysozyme-catalyzed transglycosylation reaction, and a following base-catalyzed N-demonochloroacetylation gave a chitosan oligomer mixture mainly composed of oligomers with dp > 6. Partially N-deacetylated chitin oligomers (DAC oligomers) with dp 4-12 were synthesized by the enzyme reaction using N,N',N"-tri(monochloro)acetylchitotriose and N,N',N"-triacetylchitotriose (chitin trimer) as initial substrates followed by N-demonochloroacetylation. The structures of synthetic oligomers were analyzed by 1H NMR spectroscopy, enzymatic hydrolysis and nitrous acid deamination-NaBH4 reduction treatment. The dp of synthetic oligomers was measured by MALDI TOF MS (matrix-assisted laser desorption ionization time-of-flight mass spectrometry) using per-N-acetylated derivatives. The synthetic chitosan and DAC oligomers were strong elicitors for phytoalexin induction in Pisum sativum and Phaseolus vulgaris. This chemo-enzymatic method utilizing N-acylated chitotrioses as substrates is a novel approach to the synthesis of high-molecular-weight chitosan oligomers and DAC oligomers of biological importance.


