N,N',N'',N'''-TetraacetylchitotetraoseCAS# 2706-65-2 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 2706-65-2 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
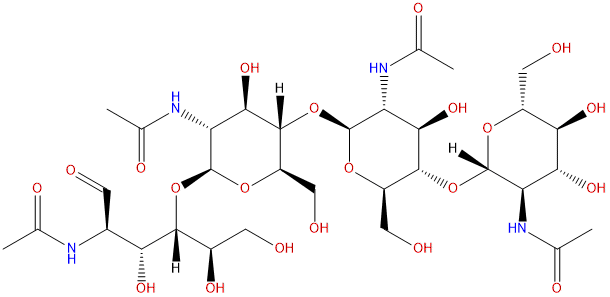
| Formula | C32H54N4O21 | M.Wt | 830.79 |
| Type of Compound | Oligoses | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

N,N',N'',N'''-Tetraacetylchitotetraose Dilution Calculator

N,N',N'',N'''-Tetraacetylchitotetraose Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2037 mL | 6.0184 mL | 12.0367 mL | 24.0735 mL | 30.0918 mL |
| 5 mM | 0.2407 mL | 1.2037 mL | 2.4073 mL | 4.8147 mL | 6.0184 mL |
| 10 mM | 0.1204 mL | 0.6018 mL | 1.2037 mL | 2.4073 mL | 3.0092 mL |
| 50 mM | 0.0241 mL | 0.1204 mL | 0.2407 mL | 0.4815 mL | 0.6018 mL |
| 100 mM | 0.012 mL | 0.0602 mL | 0.1204 mL | 0.2407 mL | 0.3009 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N,N',N'',N''',N''''-Pentaacetyl chitopentaose
Catalog No.:BCX1413
CAS No.:36467-68-2
- N,N',N'',N''',N'''',N'''''-Hexaacetyl chitohexaose
Catalog No.:BCX1412
CAS No.:38854-46-5
- Chitobiose Dihydrochloride
Catalog No.:BCX1411
CAS No.:115350-24-8
- Chitotetraose Tetrahydrochloride
Catalog No.:BCX1410
CAS No.:117399-50-5
- Chitopentaose Pentahydrochloride
Catalog No.:BCX1409
CAS No.:117467-64-8
- Chitohexaose Hexahydrochloride
Catalog No.:BCX1408
CAS No.:127171-88-4
- 5-Alpha-Hydroxy Laxogenin
Catalog No.:BCX1407
CAS No.:56786-63-1
- 1β-Methoxydiversifolin 3-O-methyl ether
Catalog No.:BCX1406
CAS No.:194474-71-0
- Emetine hydrobromide
Catalog No.:BCX1405
CAS No.:52714-87-1
- 2'-Deoxyguanosine monohydrate
Catalog No.:BCX1404
CAS No.:312693-72-4
- Gylongiposide I
Catalog No.:BCX1403
CAS No.:206876-12-2
- 4-Methoxy-2-[(6-O-β-D-xylopyranosyl-β-D-glucopyranosyl)oxy]benzaldehyde
Catalog No.:BCX1402
CAS No.:140484-68-0
- N,N',N''-Triacetylchitotriose
Catalog No.:BCX1415
CAS No.:38864-21-0
- N,N'-Diacetylchitobiose
Catalog No.:BCX1416
CAS No.:35061-50-8
- Chitopentaose Pentadecaacetate
Catalog No.:BCX1417
CAS No.:117399-52-7
- Chitotetraose tetradecaacetate
Catalog No.:BCX1418
CAS No.:53942-46-4
- Chitotriose Undecaacetate
Catalog No.:BCX1419
CAS No.:53942-45-3
- Chitobiose Octaacetate
Catalog No.:BCX1420
CAS No.:41670-99-9
- Chitotriose Trihydrochloride
Catalog No.:BCX1421
CAS No.:117436-78-9
- Chitooctaose Octahydrochloride
Catalog No.:BCX1422
CAS No.:127171-90-8
- Chitoheptaose Heptahydrochloride
Catalog No.:BCX1423
CAS No.:127171-89-5
- L-octaguluronic acid octasodium salt
Catalog No.:BCX1424
CAS No.:862694-88-0
- L-heptaguluronic acid heptasodium salt
Catalog No.:BCX1425
CAS No.:862694-87-9
- L-hexaguluronic acid hexasodium salt
Catalog No.:BCX1426
CAS No.:183668-74-8
Quantification of Protein-Ligand Interactions by Laser Electrospray Mass Spectrometry.[Pubmed:29654537]
J Am Soc Mass Spectrom. 2018 Jul;29(7):1484-1492.
Laser electrospray mass spectrometry (LEMS) measurement of the dissociation constant (K(d)) for hen egg white lysozyme (HEWL) and N,N',N''-triacetylchitotriose (NAG(3)) revealed an apparent K(d) value of 313.2 +/- 25.9 muM for the ligand titration method. Similar measurements for N,N',N'',N'''-tetraacetylchitotetraose (NAG(4)) revealed an apparent K(d) of 249.3 +/- 13.6 muM. An electrospray ionization mass spectrometry (ESI-MS) experiment determined a K(d) value of 9.8 +/- 0.6 muM. In a second LEMS approach, a calibrated measurement was used to determine a K(d) value of 6.8 +/- 1.5 muM for NAG(3). The capture efficiency of LEMS was measured to be 3.6 +/- 1.8% and is defined as the fraction of LEMS sample detected after merging with the ESI plume. When the dilution is factored into the ligand titration measurement, the adjusted K(d) value was 11.3 muM for NAG(3) and 9.0 muM for NAG(4). The calibration method for measuring K(d) developed in this study can be applied to solutions containing unknown analyte concentrations. Graphical Abstract.
Structure, Catalysis, and Inhibition of OfChi-h, the Lepidoptera-exclusive Insect Chitinase.[Pubmed:28053084]
J Biol Chem. 2017 Feb 10;292(6):2080-2088.
Chitinase-h (Chi-h) is of special interest among insect chitinases due to its exclusive distribution in lepidopteran insects and high sequence identity with bacterial and baculovirus homologs. Here OfChi-h, a Chi-h from Ostrinia furnacalis, was investigated. Crystal structures of both OfChi-h and its complex with chitoheptaose ((GlcN)(7)) reveal that OfChi-h possesses a long and asymmetric substrate binding cleft, which is a typical characteristics of a processive exo-chitinase. The structural comparison between OfChi-h and its bacterial homolog SmChiA uncovered two phenylalanine-to-tryptophan site variants in OfChi-h at subsites +2 and possibly -7. The F232W/F396W double mutant endowed SmChiA with higher hydrolytic activities toward insoluble substrates, such as insect cuticle, alpha-chitin, and chitin nanowhisker. An enzymatic assay demonstrated that OfChi-h outperformed OfChtI, an insect endo-chitinase, toward the insoluble substrates, but showed lower activity toward the soluble substrate ethylene glycol chitin. Furthermore, OfChi-h was found to be inhibited by N,N',N''-trimethylglucosamine-N,N',N'',N'''-tetraacetylchitotetraose (TMG-(GlcNAc)(4)), a substrate analog which can be degraded into TMG-(GlcNAc)(1-2) Injection of TMG-(GlcNAc)(4) into 5th-instar O. furnacalis larvae led to severe defects in pupation. This work provides insights into a molting-indispensable insect chitinase that is phylogenetically closer to bacterial chitinases than insect chitinases.
Microbial oligosaccharides differentially induce volatiles and signalling components in Medicago truncatula.[Pubmed:18534640]
Phytochemistry. 2008 Jul;69(10):2029-40.
Plants perceive biotic stimuli by recognising a multitude of different signalling compounds originating from the interacting organisms. Some of these substances represent pathogen-associated molecular patterns, which act as general elicitors of defence reactions. But also beneficial microorganisms like rhizobia take advantage of compounds structurally related to certain elicitors, i.e. Nod-factors, to communicate their presence to the host plant. In a bioassay-based study we aimed to determine to what extent distinct oligosaccharidic signals are able to elicit overlapping responses, including the emission of volatile organic compounds which is mainly considered a typical mode of inducible indirect defence against herbivores. The model legume Medicago truncatula Gaertn. was challenged with pathogen elicitors (beta-(1,3)-beta-(1,6)-glucans and N,N',N'',N'''-tetraacetylchitotetraose) and two Nod-factors, with one of them being able to induce a nodulation response in M. truncatula. Single oligosaccharidic elicitors caused the emission of volatile organic compounds, mainly sesquiterpenoids. The volatile blends detected were quite characteristic for the applied compounds, which could be pinpointed by multivariate statistical methods. As potential mediators of this response, the levels of jasmonic acid and salicylic acid were determined. Strikingly, neither of these phytohormones exhibited changing levels correlating with enhanced volatile emission. All stimuli tested caused an overproduction of reactive oxygen species, whereas nitric oxide accumulation was only effected by elicitors that were equally able to induce volatile emission. Thus, all signalling compounds tested elicited distinct reaction patterns. However, similarities between defence reactions induced by herbivory and pathogen-derived elicitors could be ascertained; but also Nod-factors were able to trigger defence-related reactions.


