Corylifol ACAS# 775351-88-7 |
- CZC24832
Catalog No.:BCC1507
CAS No.:1159824-67-5
- PI3Kγ inhibitor 1
Catalog No.:BCC4180
CAS No.:1172118-03-4
- CAL-130
Catalog No.:BCC1440
CAS No.:1431697-74-3
- A-443654
Catalog No.:BCC1321
CAS No.:552325-16-3
- CAL-101 (Idelalisib, GS-1101)
Catalog No.:BCC1270
CAS No.:870281-82-6
- BKM120
Catalog No.:BCC1279
CAS No.:944396-07-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 775351-88-7 | SDF | Download SDF |
| PubChem ID | 25056407 | Appearance | Yellowish powder |
| Formula | C25H26O4 | M.Wt | 390.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Corylifol-A; Corylinin | ||
| Solubility | DMSO : ≥ 15.4 mg/mL (39.44 mM) *"≥" means soluble, but saturation unknown. | ||
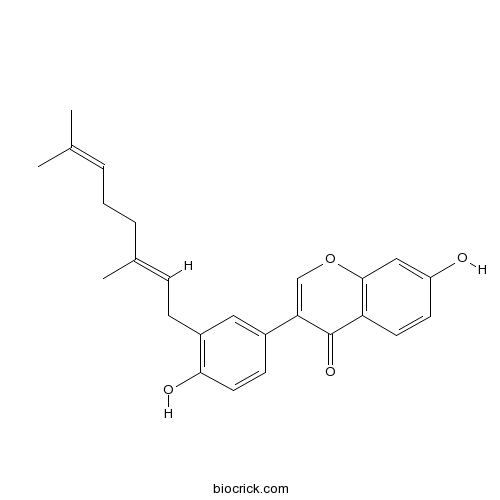
| Chemical Name | 3-[3-[(2E)-3,7-dimethylocta-2,6-dienyl]-4-hydroxyphenyl]-7-hydroxychromen-4-one | ||
| SMILES | CC(=CCCC(=CCC1=C(C=CC(=C1)C2=COC3=C(C2=O)C=CC(=C3)O)O)C)C | ||
| Standard InChIKey | ZBHUUXLHDOUMKM-REZTVBANSA-N | ||
| Standard InChI | InChI=1S/C25H26O4/c1-16(2)5-4-6-17(3)7-8-19-13-18(9-12-23(19)27)22-15-29-24-14-20(26)10-11-21(24)25(22)28/h5,7,9-15,26-27H,4,6,8H2,1-3H3/b17-7+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Corylifol A is a naturally occurring potent inhibitor of hCE2 and UDP-glucuronosyltransferase 1A1 (UGT1A1); it could be the potential uncouplers of neuronal nitric oxide synthase-postsynaptic density protein-95. Corylifol A displays cytotoxic activity against HepG2 and Hep3B hepatocellular carcinoma cell lines, with IC50 values of 4.6 and 13.5 ug/ml, respectively. Corylifol A has antiinflammatory activity, it shows an inhibitory effect on IL-6-induced STAT3 promoter activity in Hep3B cells with IC50 values of 0.81 ± 0.15 uΜ, it also inhibits STAT3 phosphorylation induced by IL-6 in Hep3B cells. |
| Targets | NOS | IL Receptor | STAT | UGT1A1 | hCE2 |
| In vitro | Enzymatic synthesis of novel corylifol A glucosides via a UDP-glycosyltransferase.[Pubmed: 28528234 ]Carbohydr Res. 2017 Jun 29;446-447:61-67.Corylifol A, a member of the isoflavone subclass of isoflavonoids, has long been considered to have various biological activities. Cytotoxic constituents from Psoralea corylifolia.[Pubmed: 23659434 ]J Asian Nat Prod Res. 2013;15(6):624-30.Bioassay directed isolation of the EtOAc extract from a traditional Chinese medicine Psoralea corylifolia resulted in the purification of two isoflavonoids, corylifols D (1) and E (2), along with four known ones. Phenolic compounds isolated from Psoralea corylifolia inhibit IL-6-induced STAT3 activation.[Pubmed: 22573369 ]Planta Med. 2012 Jun;78(9):903-6.Inhibiting interleukin-6 (IL-6) has been postulated as an effective therapy in the pathogenesis of several inflammatory diseases. |
| Kinase Assay | Fructus Psoraleae contains natural compounds with potent inhibitory effects towards human carboxylesterase 2.[Pubmed: 25596095 ]Fitoterapia. 2015 Mar;101:99-106.Fructus Psoraleae (FP) is an edible Chinese herbal which is widely used in Asia for the treatment of various diseases including asthma, diarrhea, and osteoporosis. |
| Structure Identification | J Sep Sci. 2017 Sep;40(17):3522-3534.Efficient discovery and capture of new neuronal nitric oxide synthase-postsynaptic density protein-95 uncouplers from herbal medicines using magnetic molecularly imprinted polymers as artificial antibodies.[Pubmed: 28704580 ]In the scope of stroke treatment, new neuronal nitric oxide synthase-postsynaptic density protein-95 uncouplers from herbal medicines were discovered and captured. |

Corylifol A Dilution Calculator

Corylifol A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5608 mL | 12.8041 mL | 25.6082 mL | 51.2164 mL | 64.0205 mL |
| 5 mM | 0.5122 mL | 2.5608 mL | 5.1216 mL | 10.2433 mL | 12.8041 mL |
| 10 mM | 0.2561 mL | 1.2804 mL | 2.5608 mL | 5.1216 mL | 6.402 mL |
| 50 mM | 0.0512 mL | 0.2561 mL | 0.5122 mL | 1.0243 mL | 1.2804 mL |
| 100 mM | 0.0256 mL | 0.128 mL | 0.2561 mL | 0.5122 mL | 0.6402 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Corylifol A is a phenolic compounds isolated from Psoralea corylifolia; inhibits IL-6-induced STAT3 activation and phosphorylation(IC50=0.8 uM). IC50 value: 0.8 uM [1] Target: STAT3 inhibitor Corylifol A showed an inhibitory effect on IL-6-induced STAT3 promoter activity in Hep3B cells with IC50 value of 0.81 ± 0.15 uM, also inhibited STAT3 phosphorylation induced by IL-6 in Hep3B cells [1]. Corylifol A inhibited SARA PLpro in a dose-dependent manner with IC50 ranging between 4.2 and 38.4 μM [2]. Corylifol A were found to be naturally occurring potent inhibitors of hCE2, with low Ki values ranging from 0.62μM to 3.89μM [3].
References:
[1]. Lee SW, et al. Phenolic compounds isolated from Psoralea corylifolia inhibit IL-6-induced STAT3 activation. Planta Med. 2012 Jun;78(9):903-6.
[2]. Kim DW, et al. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J Enzyme Inhib Med Chem. 2014 Feb;29(1):59-63.
[3]. Li YG, et al. Fructus Psoraleae contains natural compounds with potent inhibitory effects towards human carboxylesterase 2. Fitoterapia. 2015 Jan 13;101C:99-106. d
- Imeglimin
Catalog No.:BCC4221
CAS No.:775351-65-0
- Imeglimin hydrochloride
Catalog No.:BCC4085
CAS No.:775351-61-6
- PTC124 (Ataluren)
Catalog No.:BCC3881
CAS No.:775304-57-9
- DL-m-Tyrosine
Catalog No.:BCC3332
CAS No.:775-06-4
- Phenylpiracetam
Catalog No.:BCC1859
CAS No.:77472-70-9
- Pimobendan hydrochloride
Catalog No.:BCC4175
CAS No.:77469-98-8
- KM 11060
Catalog No.:BCC7578
CAS No.:774549-97-2
- 5-Androsten-3β-ol-17-one ethyleneketal
Catalog No.:BCC8738
CAS No.:7745-40-6
- Episesartemin A
Catalog No.:BCN7239
CAS No.:77449-31-1
- Sesartemin
Catalog No.:BCN4779
CAS No.:77394-27-5
- Drimiopsin D
Catalog No.:BCN4326
CAS No.:773850-91-2
- Drimiopsin C
Catalog No.:BCN4325
CAS No.:773850-90-1
- Corylifol B
Catalog No.:BCN3199
CAS No.:775351-90-1
- Corylifol C
Catalog No.:BCN3200
CAS No.:775351-91-2
- (20R)-Protopanaxdiol
Catalog No.:BCN1078
CAS No.:7755-01-3
- Ingenol-5,20-acetonide
Catalog No.:BCN2960
CAS No.:77573-43-4
- Ingenol-3,4:5,20-diacetonide
Catalog No.:BCN2959
CAS No.:77573-44-5
- Nestoron
Catalog No.:BCC1797
CAS No.:7759-35-5
- Isoscopoletin
Catalog No.:BCN4582
CAS No.:776-86-3
- Fmoc-HomoArg-OH
Catalog No.:BCC2645
CAS No.:776277-76-0
- 1,4,5,6-Tetrahydroxy-7,8-diprenylxanthone
Catalog No.:BCN1358
CAS No.:776325-66-7
- Toddanone
Catalog No.:BCN3430
CAS No.:77636-08-9
- Boc-D-Ala-OH
Catalog No.:BCC3049
CAS No.:7764-95-6
- Pterokaurene L3
Catalog No.:BCN4583
CAS No.:77658-38-9
Efficient discovery and capture of new neuronal nitric oxide synthase-postsynaptic density protein-95 uncouplers from herbal medicines using magnetic molecularly imprinted polymers as artificial antibodies.[Pubmed:28704580]
J Sep Sci. 2017 Sep;40(17):3522-3534.
In the scope of stroke treatment, new neuronal nitric oxide synthase-postsynaptic density protein-95 uncouplers from herbal medicines were discovered and captured. To do so, highly selective magnetic molecularly imprinted polymers with a core-shell structure were prepared as artificial antibodies. According to the results of computational simulations, we designed and synthesized various polymers with varying amounts and types of template, functional monomer, cross-linker, and solvent. Characterization and performance tests revealed that the most appropriate artificial antibodies showed uniform spherical morphologies, large adsorption capacities, fast-binding kinetics, high selectivity, and quick separation. These artificial antibodies were then used as sorbents for dispersive magnetic solid-phase extraction coupled with high-performance liquid chromatography and mass spectrometry to capture and identify structural analogs to ZL006 from extracts of Scutellariae radix, Psoraleae fructus, and Trifolium pratense. Furthermore, according to the neuroprotective effect and coimmunoprecipitation test, Baicalein, Neobavaisoflavone, Corylifol A, and Biochanin A can be the potential uncouplers of neuronal nitric oxide synthase-postsynaptic density protein-95. Therefore, this present study contributes valuable information for the discovery of neuronal nitric oxide synthase-postsynaptic density protein-95 uncouplers from herbal medicines.
Enzymatic synthesis of novel corylifol A glucosides via a UDP-glycosyltransferase.[Pubmed:28528234]
Carbohydr Res. 2017 Jun 29;446-447:61-67.
Corylifol A, a member of the isoflavone subclass of isoflavonoids, has long been considered to have various biological activities. Here, we sought to synthesize Corylifol A glucosides by the in vitro glucosylation reaction using the UDP-glycosyltransferase YjiC from Bacillus licheniformis DSM 13, and obtained two novel glucosides: Corylifol A-4',7-di-O-beta-d-glucopyranoside (1) and Corylifol A-4'-O-beta-d-glucopyranoside (2). To improve the yield of the products, the reaction time, concentration of UDP-glucose, and pH of the buffer were optimized. The Michaelis constant (Km) was calculated to be 2.88 mM, and the maximal velocity (Vmax) was calculated to be 77.32 nmol/min/mg for UDP-glycosyltransferase. Meanwhile, the water-solubility of compounds 1 and 2 was approximately 27.03 and 15.13 times higher, respectively, than that of their parent compound Corylifol A. Additionally, the Corylifol A glycosylated products exhibited the highest stability at pH 9.6 and better temperature stability than Corylifol A at 40, 60, 80 and 100 degrees C. In addition, cytotoxicity activity assays against three human tumor cell lines, only Corylifol A showed moderate anti-proliferative activity. Overall, this work demonstrates that glycosylation can enhance the water solubility and stability of promising compounds, with potential for further development and application.
Phenolic compounds isolated from Psoralea corylifolia inhibit IL-6-induced STAT3 activation.[Pubmed:22573369]
Planta Med. 2012 Jun;78(9):903-6.
Inhibiting interleukin-6 (IL-6) has been postulated as an effective therapy in the pathogenesis of several inflammatory diseases. In this study, seven flavonoids were isolated from the methanol extracts of Psoralea corylifolia by bioactivity-guided fractionation. The structures of bakuchiol (1), bavachinin (2), neobavaisoflavone (3), Corylifol A (4), corylin (5), isobavachalcon (6), and bavachin (7) were determined by spectroscopic analysis (1H-, 13C- NMR and MS). We demonstrated that compounds 1-7 showed an inhibitory effect on IL-6-induced STAT3 promoter activity in Hep3B cells with IC50 values of 4.57 +/- 0.45, 3.02 +/- 0.53, 2.77 +/- 0.02, 0.81 +/- 0.15, 1.37 +/- 0.45, 2.45 +/- 0.13, and 4.89 +/- 0.05 microMu, respectively. These compounds also inhibited STAT3 phosphorylation induced by IL-6 in Hep3B cells. Overall, several flavonoids from P. corylifolia might be useful remedies for treating inflammatory diseases by inhibiting IL-6-induced STAT3 activation and phosphorylation.
Fructus Psoraleae contains natural compounds with potent inhibitory effects towards human carboxylesterase 2.[Pubmed:25596095]
Fitoterapia. 2015 Mar;101:99-106.
Fructus Psoraleae (FP) is an edible Chinese herbal which is widely used in Asia for the treatment of various diseases including asthma, diarrhea, and osteoporosis. This study aimed to investigate the inhibitory effects of the crude ethanol extract from FP on human carboxylesterase 2 (hCE2), as well as to identity and characterize the naturally occurring inhibitors of hCE2 in FP. Our results demonstrated that the ethanol extract of FP displayed potent inhibitory effects towards hCE2, while five major bioactive constitutes in FP were efficiently identified by LC-DAD-ESI-MS/MS, with the aid of LC-based activity profiling. The identified bioactive compounds including neobavaisoflavone, isobavachalcone, bavachinin, Corylifol A and bakuchiol were found to be naturally occurring potent inhibitors of hCE2, with low Ki values ranging from 0.62muM to 3.89muM. This is the first report of the chemical constitutes in FP as potent inhibitors of hCE2.
Cytotoxic constituents from Psoralea corylifolia.[Pubmed:23659434]
J Asian Nat Prod Res. 2013;15(6):624-30.
Bioassay directed isolation of the EtOAc extract from a traditional Chinese medicine Psoralea corylifolia resulted in the purification of two isoflavonoids, corylifols D (1) and E (2), along with four known ones. The structures of 1 and 2 were determined by extensive 1D and 2D NMR and MS data analyses. When tested against HepG2 and Hep3B hepatocellular carcinoma cell lines, Corylifol A (4) displayed IC50 values of 4.6 and 13.5 mug/ml, respectively.


