Coumalic acidCAS# 500-05-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 500-05-0 | SDF | Download SDF |
| PubChem ID | 68141.0 | Appearance | Powder |
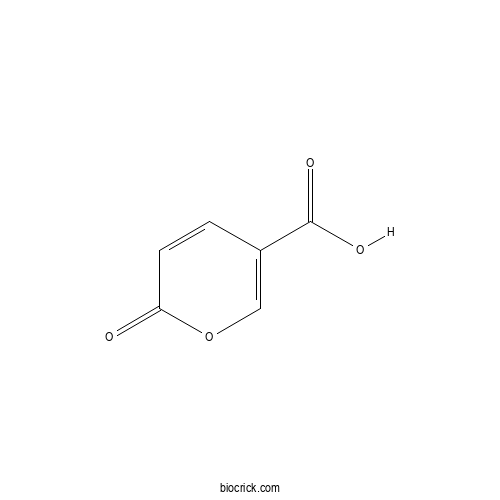
| Formula | C6H4O4 | M.Wt | 140.09 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 6-oxopyran-3-carboxylic acid | ||
| SMILES | C1=CC(=O)OC=C1C(=O)O | ||
| Standard InChIKey | ORGPJDKNYMVLFL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H4O4/c7-5-2-1-4(3-10-5)6(8)9/h1-3H,(H,8,9) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Coumalic acid Dilution Calculator

Coumalic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.1383 mL | 35.6913 mL | 71.3827 mL | 142.7654 mL | 178.4567 mL |
| 5 mM | 1.4277 mL | 7.1383 mL | 14.2765 mL | 28.5531 mL | 35.6913 mL |
| 10 mM | 0.7138 mL | 3.5691 mL | 7.1383 mL | 14.2765 mL | 17.8457 mL |
| 50 mM | 0.1428 mL | 0.7138 mL | 1.4277 mL | 2.8553 mL | 3.5691 mL |
| 100 mM | 0.0714 mL | 0.3569 mL | 0.7138 mL | 1.4277 mL | 1.7846 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dehydrosulphurenic acid
Catalog No.:BCX0651
CAS No.:175615-56-2
- 4-Ethoxybenzyl alcohol
Catalog No.:BCX0650
CAS No.:6214-44-4
- D-Tartaric acid
Catalog No.:BCX0649
CAS No.:147-71-7
- Coniferylaldehydel
Catalog No.:BCX0648
CAS No.:458-36-6
- Genistein 8-C-glucoside
Catalog No.:BCX0647
CAS No.:66026-80-0
- Phytosphingosine
Catalog No.:BCX0646
CAS No.:554-62-1
- Apigenin-6-C-β-D-xylopyranosyl-8-C-α-L-arabinopyranoside
Catalog No.:BCX0645
CAS No.:85700-46-5
- L-xylose
Catalog No.:BCX0644
CAS No.:609-06-3
- 8-Epi-Loganic acid-6'-O-β-D-glucoside
Catalog No.:BCX0643
CAS No.:176226-39-4
- Isokadsuranin
Catalog No.:BCX0642
CAS No.:82467-52-5
- 2-O-β-D-Glucopyranosyl-L-ascorbic acid
Catalog No.:BCX0641
CAS No.:562043-82-7
- Gymnoside IX
Catalog No.:BCX0640
CAS No.:898827-00-4
- 3-Indoleacetamide
Catalog No.:BCX0653
CAS No.:879-37-8
- 8,9-epoxy-3-isobutyryloxy-10-(2-methylbutanoyl)thymol
Catalog No.:BCX0654
CAS No.:22518-07-6
- 4-O-galloylalbiflorin
Catalog No.:BCX0655
CAS No.:1201580-97-3
- Glycyroside
Catalog No.:BCX0656
CAS No.:125310-04-5
- Acid Red 73
Catalog No.:BCX0657
CAS No.:5413-75-2
- 3-O-Coumaroylquinic acid
Catalog No.:BCX0658
CAS No.:87099-71-6
- 6'-O-galloylalbiflorin
Catalog No.:BCX0659
CAS No.:929042-36-4
- Filbertone
Catalog No.:BCX0660
CAS No.:81925-81-7
- Azorubin
Catalog No.:BCX0661
CAS No.:3567-69-9
- Ophiopogonside A
Catalog No.:BCX0662
CAS No.:2423917-90-0
- 6''-O-apiosyl-Visammioside
Catalog No.:BCX0663
CAS No.:2254096-97-2
- Isotoosendanin
Catalog No.:BCX0664
CAS No.:97871-44-8
Colonization of root endophytic fungus Serendipita indica improves drought tolerance of Pinus taeda seedlings by regulating metabolome and proteome.[Pubmed:38559354]
Front Microbiol. 2024 Mar 15;15:1294833.
Pinus taeda is an important forest tree species for plantations because of its rapid growth and high yield of oleoresins. Although P. taeda plantations distribute in warm and wet southern China, drought, sometime serious and long time, often occurs in the region. To explore drought tolerance of P. taeda and usage of beneficial microorganisms, P. taeda seedlings were planted in pots and were inoculated with root endophytic fungus Serendipita indica and finally were treated with drought stress for 53 d. Metabolome and proteome of their needles were analyzed. The results showed that S. indica inoculation of P. taeda seedlings under drought stress caused great changes in levels of some metabolites in their needles, especially some flavonoids and organic acids. Among them, the levels of eriocitrin, trans-aconitic acid, vitamin C, uric acid, alpha-ketoglutaric acid, vitamin A, stachydrine, Coumalic acid, itaconic acid, calceolarioside B, 2-oxoglutaric acid, and citric acid were upregulated more than three times in inoculated seedlings under drought stress, compared to those of non-inoculated seedlings under drought stress. KEGG analysis showed that some pathways were enriched in inoculated seedlings under drought stress, such as flavonoid biosynthesis, ascorbate and aldarate metabolism, C5-branched dibasic acid metabolism. Proteome analysis revealed some specific differential proteins. Two proteins, namely, H9X056 and H9VDW5, only appeared in the needles of inoculated seedlings under drought stress. The protein H9VNE7 was upregulated more than 11.0 times as that of non-inoculated seedlings under drought stress. In addition, S. indica inoculation increased enrichment of water deficient-inducible proteins (such as LP3-1, LP3-2, LP3-3, and dehydrins) and those involved in ribosomal structures (such as A0A385JF23). Meanwhile, under drought stress, the inoculation caused great changes in biosynthesis and metabolism pathways, mainly including phenylpropanoid biosynthesis, cutin, suberine and wax biosynthesis, and 2-oxocarboxylic acid metabolism. In addition, there were positive relationships between accumulation of some metabolites and enrichment of proteins in P. taeda under drought stress. Altogether, our results showed great changes in metabolome and proteome in inoculated seedlings under drought stress and provided a guideline to further study functions of metabolites and proteins, especially those related to drought stress.
Transcriptional Stages of Conidia Germination and Associated Genes in Aspergillus flavus: An Essential Role for Redox Genes.[Pubmed:36006223]
Toxins (Basel). 2022 Aug 18;14(8):560.
Aflatoxin is a threatening mycotoxin primarily present in the agricultural environment, especially in food and feedstuff, and poses significant global health risks. Aflatoxins are produced mainly by Aspergillus flavus. Conidia germination is the first step for A. flavus development. In this study, the transcriptome of A. flavus conidia was analyzed at three different stages of conidia germination, which were characterized by two different microscopes. Dormant conidia grew isotropically with the cell size increasing up to 5 h of after being inoculated in a liquid medium. Conidia changed towards polarized growth from 5 to 10 h of germination, during which germ tubes formed. Moreover, transcriptome analyses revealed that a larger number of genes changed in the isotropic growth stages compared to polarized growth, with 1910 differentially expressed genes (DEGs) up-regulated and 969 DEGs down-regulated in isotropic growth. GO and KEGG pathway analyses and pathway enrichment demonstrated that, in the isotropic growth stage, the top three pathways were translation, amino acid and carbohydrate metabolism. The ribosome was a key pathway in translation, as RPS28e, RPL53 and RPL36e were the top three DEGs. For polarized growth stage, lipid metabolism, amino acid metabolism and carbohydrate metabolism were the top three most active pathways. POX1 from alpha-linolenic acid metabolism was a DEG in lipid metabolism as well. Genes related to the antioxidant system were crucial for conidia germination. Furthermore, RT-PCR results showed the same trends as the transcriptome for redox genes, and essential oils have a significant inhibitory effect on germination rate and redox gene expression. Therefore, redox genes play an important role during germination, and the disruption of redox genes is involved in the mechanism of action of Coumalic acid and geraniol against A. flavus spore germination.
Novel Insights on Human Carbonic Anhydrase Inhibitors Based on Coumalic Acid: Design, Synthesis, Molecular Modeling Investigation, and Biological Studies.[Pubmed:35887299]
Int J Mol Sci. 2022 Jul 19;23(14):7950.
Human carbonic anhydrase (hCA, EC 4.2.1.1) isoforms IX and XII are overexpressed in solid hypoxic tumors, and they are considered as prognostic tools and therapeutic targets for cancer. Based on a molecular simplification of the well-known coumarin scaffold, we developed a new series of derivatives of the pyran-2-one core. The new compounds are endowed with potent and selective inhibitory activity against the tumor-related hCA isoforms IX and XII, in the low nanomolar range, whereas they are inactive against the two cytosolic off-targets hCA I and II. The compounds exhibiting the best hCA inhibition were further investigated against the breast adenocarcinoma cell line (MCF7) in hypoxic conditions, evaluating their ability to eventually synergize with doxorubicin. The compounds' biocompatibility on healthy cells was also tested and confirmed on Human Gingival Fibroblasts (HGFs). Furthermore, the possible binding mode of all compounds to the active site of the tumor-associated human CA IX was investigated by computational techniques which predicted the binding conformations and the persistency of binding poses within the active site of the enzyme, furnishing relevant data for the design of tight binding inhibitors.
Discovery of the active compounds of Smilacis Glabrae Rhizoma by utilizing the relationship between the individual differences in blood drug concentration and the pharmacological effect in rats.[Pubmed:32325179]
J Ethnopharmacol. 2020 Aug 10;258:112886.
ETHNOPHARMACOLOGICAL RELEVANCE: This study addresses the rapid discovery of the active compounds (the original constituents and/or metabolites) of a traditional Chinese drug, Smilacis Glabrae Rhizoma (SGR). AIM OF THE STUDY: The aim of this study was to develop a new method to find out the active compounds of traditional drugs in vivo. MATERIALS AND METHODS: A method was established to discover and identify the potential active compounds in drug-containing plasma from rats that were orally administered SGR extract, utilizing the relationship between the individual differences in blood drug concentrations in the rats and the resulting differences in pharmacological effect, and the method was denoted as the RID-PE method. For this method, we used high-performance liquid chromatography with a diode array detector combined with electrospray ionization ion trap time-of-flight multistage mass spectrometry (LC-MS(n)) to identify the compounds (the original constituents and metabolites) and to determine the peak areas of the compounds in drug-containing plasma following SGR treatment. The anti-inflammatory effect of SGR was evaluated using a carrageenan-induced inflammatory rat model. According to the percent inhibition of paw edema in each model rat (14 rats total) orally administered SGR extract, the plasma samples from the rats were sorted and divided into 7 groups. Each group consisted of two plasma samples, and their percent inhibition of paw edema were similar to each other. We performed an LC-MS(n) analysis on 3 plasma groups, which showed large differences in the inhibition rates, with percent inhibitions of 92.7%, 72.4% and 38.4%. The correlation coefficients (r) between the peak area of each compound and the pharmacological effect (inhibition ratio) of SGR in the three groups were analyzed using SPSS software. When the correlation coefficients of the compounds are greater than 0.8 (0.8 < r Coumalic acid, resveratrol-3'-O-glucuronide (RG, isomer of M2 or M3), 3'-O-methyl-(+)-epicatechin-4'-O-glucuronide (CA-1, isomer of M16), 4'-O-methyl-(+)-epicatechin-3'-O-glucuronide (CA-2, isomer of M16), 4'-O-methyl-(+)-epicatechin-7-O-glucuronide (CA-3, isomer of M16) and 3'-O-methyl-(+)-epicatechin-7-O-glucuronide (CA-4, isomer of M16). In addition, four isomers (CA-1-CA-4) were reported to have anti-inflammatory effects for the first time, and CA-3 was a new compound. CONCLUSIONS: The RID-PE method can be used to discover and identify the active constituents and metabolites of SGR systematically and in vivo. Furthermore, these findings enhance our understanding of the metabolism and effective forms of SGR.
Interactions of beta-Conglycinin (7S) with Different Phenolic Acids-Impact on Structural Characteristics and Proteolytic Degradation of Proteins.[Pubmed:27706090]
Int J Mol Sci. 2016 Oct 2;17(10):1671.
p-Coumalic acid (PCA), caffeic acid (CA), gallic acid (GA) and chlorogenic acid (CGA) are the major phenolic acids that co-exist with soy protein components in foodstuffs. Surprisingly, there are only a handful of reports that describe their interaction with beta-Conglycinin (7S), a major soy protein. In this report, we investigated the interaction between phenolic acids and soy protein 7S and observed an interaction between each of these phenolic acids and soy protein 7S, which was carried out by binding. Further analysis revealed that the binding activity of the phenolic acids was structure dependent. Here, the binding affinity of CA and GA towards 7S was found to be stronger than that of PCA, because CA and GA have one more hydroxyl group. Interestingly, the binding of phenolic acids with soy protein 7S did not affect protein digestion by pepsin and trypsin. These findings aid our understanding of the relationship between different phenolic acids and proteins in complex food systems.
[Chemical constituents from safflower injection and their bioactivity].[Pubmed:25509295]
Zhongguo Zhong Yao Za Zhi. 2014 Aug;39(16):3102-6.
The chemical constituents of Safflower injection were isolated and purified by polyamide, silica gel, Sephadex LH-20, ODS column chromatographies and preparative HPLC. As a result, sixteen compounds have been isolated. Based on the spectral data analysis, their structures were elucidated as scutellarin (1), kaempferol-3-O-beta-rutinoside(2), hydroxysafflor yellow A(3), rutin (4), Coumalic acid(5), adenosine(6), syringoside(7), (3E)-4-(4'-hydroxyphenyl)-3-buten-2-one(8), (8Z)-decaene-4, 6-diyne-1-Obeta-D-glucopyranoside(9), 4-hydroxybenzaldehyde (10), (2E, 8E) -tetradecadiene-4, 6-diyne-1, 12, 14-triol-1-O-beta-D-glucopyranoside (11), kaem-pferol-3-O-beta-sophorose (12), uridine (13), roseoside (14), cinnamic acid (15), and kaempferol (16). Compounds 1,2,7,9,11 and 12 were isolated from the Safflower injection for the first time. The anti-platelet aggregation activities of the isolated compounds were assayed. The results indicated all tested compounds exhibited potent activity except for 5, while 2, 3, 9 and 12 showed strong activity against platelet aggregation.
Inhibition of Orobanche crenata seed germination and radicle growth by allelochemicals identified in cereals.[Pubmed:24044614]
J Agric Food Chem. 2013 Oct 16;61(41):9797-803.
Orobanche crenata is a parasitic weed that causes severe yield losses in important grain and forage legume crops. Cereals have been reported to inhibit O. crenata parasitism when grown intercropped with susceptible legumes, but the responsible metabolites have not been identified. A number of metabolites have been reported in cereals that have allelopathic properties against weeds, pests, and pathogens. We tested the effect of several allelochemicals identified in cereals on O. crenata seed germination and radicle development. We found that 2-benzoxazolinone, its derivative 6-chloroacetyl-2-benzoxazolinone, and scopoletin significantly inhibited O. crenata seed germination. Benzoxazolinones, l-tryptophan, and Coumalic acid caused the stronger inhibition of radicle growth. Also, other metabolites reduced radicle length, this inhibition being dose-dependent. Only scopoletin caused cell necrotic-like darkening in the young radicles. Prospects for their application to parasitic weed management are discussed.
The inhibiting activity of areca inflorescence extracts on human low density lipoprotein oxidation induced by cupric ion.[Pubmed:21942744]
Int J Food Sci Nutr. 2012 Mar;63(2):236-41.
The oxidative modification of human low density lipoprotein (LDL) plays a significant role in atherosclerosis. In this study, the inhibiting activity of areca inflorescence extracts (AIEs) on LDL oxidation was investigated by an in vitro study with Trolox as the standard antioxidant. The kinetics of LDL oxidation, thiobarbituric acid reactive substances assay, ferric-reducing antioxidant power assay and copper chelation assay were also evaluated to assess the antioxidant activities of AIEs, and the results revealed that AIEs could delay the lag time and inhibit the formation of malondialdehyde in the process of LDL peroxidation induced by Cu(2+). The boiled water extract displayed the highest antioxidant activity compared with the ambient water extract and ethanol extract. The total phenolic contents and phenolic components of AIEs were also measured by high performance liquid chromatography method. Epicatechin, gallic acid and Coumalic acid were the primary phenolic acids in AIEs.
Stimulation by caffeic acid, coumalic acid, and corilagin of the germination of resting spores of the clubroot pathogen Plasmodiophora brassicae.[Pubmed:12619690]
Biosci Biotechnol Biochem. 2003 Jan;67(1):170-3.
Some chemicals were examined for their effects on the germination of resting spores of the clubroot pathogen Plasmodiophora brassicae, and on the control of clubroots in Chinese cabbage. Caffeic acid, Coumalic acid, and corilagin stimulated the germination of Plasmodiophora spores and prevented the formation of clubroots in Chinese cabbage. Clubroot might be controlled by agents with germination-stimulating effects.
Time-dependent inactivation of chick-embryo prolyl 4-hydroxylase by coumalic acid. Evidence for a syncatalytic mechanism.[Pubmed:3036081]
Biochem J. 1987 Feb 15;242(1):163-9.
From the structure-activity relationships of known competitive inhibitors, Coumalic acid (2-oxo-1,2H-pyran-5-carboxylic acid) was deduced to be a potential syncatalytic inhibitor for chick-embryo prolyl 4-hydroxylase. The compound caused time-dependent inactivation, the reaction rate being first-order. The inactivation constant was 0.094 min-1, the Ki 17 mM and the bimolecular rate constant 0.09 M-1 X S-1. Human prolyl 4-hydroxylase and chick embryo lysyl hydroxylase were also inactivated, though to a lesser extent. Inactivation could be prevented by adding high concentrations of 2-oxoglutarate or its competitive analogues to the reaction mixture. In Lineweaver-Burk kinetics, Coumalic acid displayed S-parabolic competitive inhibition with respect to 2-oxoglutarate. The inactivation reaction had cofactor requirements similar to those for the decarboxylation of 2-oxoglutarate. Enzymic activity was partially preserved in the absence of iron, but the rescue was incomplete, owing to decreased stability of the enzyme under this condition. Coumalic acid also decreased the electrophoretic mobility of the alpha-subunit, but the beta-subunit was not affected. Prolonged incubation of Coumalic acid above pH 6.8 led to loss of its inactivating potency, owing to hydrolysis. It is concluded that the inactivation of prolyl 4-hydroxylase by Coumalic acid is due to a syncatalytic mechanism. The data also suggest that the 2-oxoglutarate-binding site of the enzyme is located within the alpha-subunit.


