FilbertoneCAS# 81925-81-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 81925-81-7 | SDF | Download SDF |
| PubChem ID | 5362588.0 | Appearance | Powder |
| Formula | C8H14O | M.Wt | 126.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
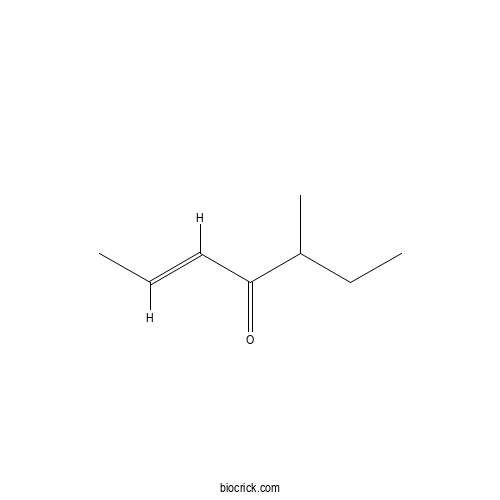
| Chemical Name | (E)-5-methylhept-2-en-4-one | ||
| SMILES | CCC(C)C(=O)C=CC | ||
| Standard InChIKey | ARJWAURHQDJJAC-GQCTYLIASA-N | ||
| Standard InChI | InChI=1S/C8H14O/c1-4-6-8(9)7(3)5-2/h4,6-7H,5H2,1-3H3/b6-4+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Filbertone Dilution Calculator

Filbertone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.9239 mL | 39.6197 mL | 79.2393 mL | 158.4786 mL | 198.0983 mL |
| 5 mM | 1.5848 mL | 7.9239 mL | 15.8479 mL | 31.6957 mL | 39.6197 mL |
| 10 mM | 0.7924 mL | 3.962 mL | 7.9239 mL | 15.8479 mL | 19.8098 mL |
| 50 mM | 0.1585 mL | 0.7924 mL | 1.5848 mL | 3.1696 mL | 3.962 mL |
| 100 mM | 0.0792 mL | 0.3962 mL | 0.7924 mL | 1.5848 mL | 1.981 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6'-O-galloylalbiflorin
Catalog No.:BCX0659
CAS No.:929042-36-4
- 3-O-Coumaroylquinic acid
Catalog No.:BCX0658
CAS No.:87099-71-6
- Acid Red 73
Catalog No.:BCX0657
CAS No.:5413-75-2
- Glycyroside
Catalog No.:BCX0656
CAS No.:125310-04-5
- 4-O-galloylalbiflorin
Catalog No.:BCX0655
CAS No.:1201580-97-3
- 8,9-epoxy-3-isobutyryloxy-10-(2-methylbutanoyl)thymol
Catalog No.:BCX0654
CAS No.:22518-07-6
- 3-Indoleacetamide
Catalog No.:BCX0653
CAS No.:879-37-8
- Coumalic acid
Catalog No.:BCX0652
CAS No.:500-05-0
- Dehydrosulphurenic acid
Catalog No.:BCX0651
CAS No.:175615-56-2
- 4-Ethoxybenzyl alcohol
Catalog No.:BCX0650
CAS No.:6214-44-4
- D-Tartaric acid
Catalog No.:BCX0649
CAS No.:147-71-7
- Coniferylaldehydel
Catalog No.:BCX0648
CAS No.:458-36-6
- Azorubin
Catalog No.:BCX0661
CAS No.:3567-69-9
- Ophiopogonside A
Catalog No.:BCX0662
CAS No.:2423917-90-0
- 6''-O-apiosyl-Visammioside
Catalog No.:BCX0663
CAS No.:2254096-97-2
- Isotoosendanin
Catalog No.:BCX0664
CAS No.:97871-44-8
- N-benzylpentadecanamide
Catalog No.:BCX0665
CAS No.:1572037-13-8
- Safranal
Catalog No.:BCX0666
CAS No.:116-26-7
- Apigenin-7-diglucuronide
Catalog No.:BCX0667
CAS No.:74696-01-8
- Trans-Emodin bianthrone
Catalog No.:BCX0668
CAS No.:61281-20-7
- Bigelovin
Catalog No.:BCX0669
CAS No.:3668-14-2
- Ankaflavin
Catalog No.:BCX0670
CAS No.:50980-32-0
- Ergolide
Catalog No.:BCX0671
CAS No.:54999-07-4
- Sorbitol
Catalog No.:BCX0672
CAS No.:50-70-4
In vivo aroma release and perception of composite foods using nose space PTR-ToF-MS analysis with Temporal-Check-All-That-Apply.[Pubmed:37087281]
Food Res Int. 2023 May;167:112726.
In vivo aroma release and perception of complex food matrices have been underexplored. The aims of this study were to investigate the effects of (i) fat and sugar content of chocolate-hazelnut spreads on in vivo aroma release and perception and (ii) carrier addition (bread, wafer) on in vivo aroma release and perception of chocolate-hazelnut spread using dynamic nose space analysis (PTR-ToF-MS) and dynamic sensory analysis (TCATA). Carriers were combined with spreads varying in fat and sugar content and were spiked with five volatile organic compounds (benzaldehyde, Filbertone, 2-methylpyrazine, delta-dodecalactone, isovaleraldehyde). TCATA profiles from a consumer panel without in vivo nose space analysis (n = 72) and a trained panel performing in vivo nose space analysis (n = 8, triplicate) were compared. TCATA profiles of the spread-carrier combinations obtained by both panels showed similarly that attributes related to the carriers were perceived at the beginning of consumption, whereas attributes related to the spreads were perceived after swallowing. Significant (p < 0.05) and small differences were observed for the attributes cocoa, creamy, milky, sticky and toffee between both panels. In the evaluated reformulation range, fat and sugar content of chocolate-hazelnut spreads had only a limited effect on in vivo aroma release and perception. In contrast, addition of carriers strongly affected in vivo aroma release and perception for all target molecules. The addition of carriers to spreads generally increased aroma release (duration and intensity of aroma release) and decreased aroma perception. The addition of carriers generally reduced the time to reach maximum intensity compared to when spreads were eaten alone for the five volatile organic compounds while perception decreased. We conclude that the strong effect of carrier addition on in vivo aroma release and perception of chocolate-hazelnut spreads highlights the importance of investigating toppings/spreads accompanied with carriers rather than in isolation.
Activation of ROS-PERK-TFEB by filbertone ameliorates neurodegenerative diseases via enhancing the autophagy-lysosomal pathway.[Pubmed:36958418]
J Nutr Biochem. 2023 Aug;118:109325.
The molecular mechanisms underlying the pathogenesis of neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease (PD), and Huntington's disease remain enigmatic, resulting in an unmet need for therapeutics development. Here, we suggest that Filbertone, a key flavor compound found in the fruits of hazel trees of the genus Corylus, can ameliorate PD via lowering the abundance of aggregated alpha-synuclein. We previously reported that inhibition of hypothalamic inflammation by Filbertone is mediated by suppression of nuclear factor kappa-B. Here, we report that Filbertone activates PERK through mitochondrial reactive oxygen species production, resulting in the increased nuclear translocation of transcription factor-EB in SH-SY5Y human neuroblastoma cells. TFEB activation by Filbertone promotes the autophagy-lysosomal pathway, which in turn alleviates the accumulation of alpha-synuclein. We also demonstrate that Filbertone prevented the loss of dopaminergic neurons in the substantia nigra and striatum of mice on high-fat diet. Filbertone treatment also reduced high-fat diet-induced alpha-synuclein accumulation through upregulation of the autophagy-lysosomal pathway. In addition, Filbertone improved behavioral abnormalities (i.e., latency time to fall and decrease of running distance) in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced PD murine model. In conclusion, Filbertone may show promise as a potential therapeutic for neurodegenerative disease.
Corylus avellana L. Natural Signature: Chiral Recognition of Selected Informative Components in the Volatilome of High-Quality Hazelnuts.[Pubmed:35548269]
Front Plant Sci. 2022 Apr 25;13:844711.
The volatile fraction of plant-based foods provides useful functional information concerning sample-related variables such as plant genotype and phenotype expression, pedoclimatic and harvest conditions, transformation/processing technologies, and can be informative about the sensory quality. In this respect, the enantiomeric recognition of the chiral compounds increases the level of information in profiling studies, being the biosynthesis of native compounds often stereo-guided. Chiral native volatiles mostly show an enantiomeric excess that enables origin authentication or support correlation studies between chemical patterns and sensory profiles. This study focuses, for the first time, on the enantiomeric composition of a large set of chiral compounds within the complex volatilome of Corylus avellana L. belonging to different cultivars (Tonda Gentile Romana, Tonda Gentile Trilobata, Anakliuri) and harvested in different geographical areas (Italian and Georgian). Besides native components profiled in raw kernels, volatiles formed after technological treatment (i.e., roasting) are also considered. Headspace solid-phase microextraction combined with enantioselective gas chromatography-mass spectrometry enables the accurate tracking and annotation of about 150 compounds across many samples. The results show that chiral compounds have diagnostic distribution patterns within hazelnut volatilome with cultivar and harvest region playing the major role. Moreover, being some of these chiral molecules also key-aromas, their distribution has a decisive impact on the sensory properties of the product. In particular, the enantiomeric composition of (E)-5-methyl-2-hepten-4-one (Filbertone) resulted to be discriminant for origin authentication. The enantiomeric distribution showed, according to literature, an excess of the (S)-enantiomer in both raw and roasted samples volatilome with larger differences in raw samples. The amount of both (R) and (S)-Filbertone increases during roasting; the most marked increase for (R)-enantiomer is observed in Italian samples, thus supporting evidence of better hedonic properties and more pleasant odor and aroma.
Filbertone Ameliorates Adiposity in Mice Fed a High-Fat Diet via Activation of cAMP Signaling.[Pubmed:31366045]
Nutrients. 2019 Jul 30;11(8):1749.
The aim of this research was to estimate the preventive effects of Filbertone, the main flavor compound in hazelnuts, on lipid accumulation in the adipose tissue of mice fed a high-fat diet (HFD) and to reveal the underlying molecular mechanisms. Male C57BL/6N mice were fed chow, a HFD, or a 0.025% Filbertone-supplemented HFD for 14 weeks. We found that Filbertone supplementation resulted in significant reductions in body weight gain and lipid accumulation in adipose tissue, with parallel improvements in plasma lipid levels (triglycerides, total cholesterol, and free fatty acids) and proinflammatory cytokines (interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-alpha)). Molecular analysis revealed that Filbertone treatment led to reprogramming of metabolic signatures in the cyclic adenosine monophosphate (cAMP) pathway. Filbertone supplementation significantly increased the cAMP level and increased downstream protein kinase A catalytic subunit (PKA) signaling in mouse adipose tissue. The mRNA level of adipogenesis-related genes was downregulated in the adipose tissue of Filbertone-fed mice compared to control mice fed the HFD alone. Furthermore, Filbertone treatment elevated the expression of thermogenic genes in mouse adipose tissue. Filbertone reduced intracellular lipid accumulation and increased the oxygen consumption rate in 3T3-L1 cells and these Filbertone-induced changes were abrogated by the adenylate cyclases (ADCY) inhibitor. Taken together, our results suggest that the beneficial effects of Filbertone on lipid accumulation may be associated with the activation of cAMP signaling.
Filbertone: A Review.[Pubmed:30303012]
J Agric Food Chem. 2018 Oct 31;66(43):11221-11226.
This comprehensive review of Filbertone, a principal flavor compound of hazelnut, evaluates the current state of the art of all relevant aspects of the title molecule: its occurrence and properties, laboratory preparation and bulk synthesis, analytical issues regarding stereochemistry and purity, sensory evaluation, and practical uses. Comparisons are made between different synthetic approaches, and a critical assessment of various applications is presented.
Determination of filbertone in spiked olive oil samples using headspace-programmed temperature vaporization-gas chromatography-mass spectrometry.[Pubmed:19396589]
Anal Bioanal Chem. 2009 Jul;394(5):1463-70.
A sensitive method for the fast analysis of Filbertone in spiked olive oil samples is presented. The applicability of a headspace (HS) autosampler in combination with a gas chromatograph (GC) equipped with a programmable temperature vaporizer (PTV) and a mass spectrometric (MS) detector is explored. A modular accelerated column heater (MACH) was used to control the temperature of the capillary gas chromatography column. This module can be heated and cooled very rapidly, shortening total analysis cycle times to a considerable extent. The proposed method does not require any previous analyte extraction, filtration and preconcentration step, as in most methods described to date. Sample preparation is reduced to placing the olive oil sample in the vial. This reduces the analysis time and the experimental errors associated with this step of the analytical process. By using headspace generation, the volatiles of the sample are analysed without interference by the non-volatile matrix, and by using injection in solvent-vent mode at the PTV inlet, most of the compounds that are more volatile than Filbertone are purged and the matrix effect is minimised. Use of a liner packed with Tenax-TA allowed the compound of interest to be retained during the venting process. The limits of detection and quantification were as low as 0.27 and 0.83 microg/L, respectively, and precision (measured as the relative standard deviation) was 5.7%. The method was applied to the determination of Filbertone in spiked olive oil samples and the results revealed the good accuracy obtained with the method.
Semipreparative-scale gas-chromatographic separation of filbertone enantiomers.[Pubmed:16605089]
J Sep Sci. 2006 Mar;29(5):691-4.
The enantioselectivity of heptakis(2,3-di-O-acetyl-6-O-tert-butyldimethylsilyl-beta-CD) toward racemic Filbertone (E-5-methyl-hep-2-en-4-one) was studied by performing the chiral separation on a capillary column, a thick-film wide-bore column and a semipreparative column. The semipreparative enantioseparation of Filbertone was achieved at 80 degrees C by using a packed column providing (R)- and (S)-enantiomers of Filbertone with ee 90 and 96%, respectively. The isolated enantiomers (approximately 250 microg each, ee = 90-96%) may be used for studies on the relationship of chirality and biological activity by olfactory screening and toxicological studies.
Solid-phase microextraction for studies on the enantiomeric composition of filbertone in hazelnut oils.[Pubmed:12696926]
J Agric Food Chem. 2003 Apr 23;51(9):2496-500.
The enantiomeric distribution of Filbertone was determined in unroasted and roasted hazelnut oils of different geographical origins by using solid-phase microextraction (SPME) and capillary gas chromatography. An optimization procedure including SPME fiber, extraction time, exposure temperature, and sample volume enabled the best conditions to be selected. Under the optimized conditions, detection limits were in the micrograms per liter level for both enantiomers of Filbertone with relative standard deviation values of 7.1 and 4.9% for R-Filbertone and S-Filbertone, respectively. The proposed approach allowed the rapid determination of the enantiomeric composition of Filbertone and demonstrated that its variability is an inherent property of the natural compound. Analysis of two batches of hazelnut oils obtained from either unroasted or roasted hazelnuts showed, in general, significantly higher amounts of Filbertone in roasted hazelnut oils.


