ErgolideCAS# 54999-07-4 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 54999-07-4 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
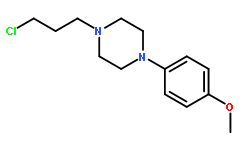
| Formula | C14H21N2OCl | M.Wt | 268.78 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ergolide Dilution Calculator

Ergolide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7205 mL | 18.6026 mL | 37.2051 mL | 74.4103 mL | 93.0129 mL |
| 5 mM | 0.7441 mL | 3.7205 mL | 7.441 mL | 14.8821 mL | 18.6026 mL |
| 10 mM | 0.3721 mL | 1.8603 mL | 3.7205 mL | 7.441 mL | 9.3013 mL |
| 50 mM | 0.0744 mL | 0.3721 mL | 0.7441 mL | 1.4882 mL | 1.8603 mL |
| 100 mM | 0.0372 mL | 0.186 mL | 0.3721 mL | 0.7441 mL | 0.9301 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ankaflavin
Catalog No.:BCX0670
CAS No.:50980-32-0
- Bigelovin
Catalog No.:BCX0669
CAS No.:3668-14-2
- Trans-Emodin bianthrone
Catalog No.:BCX0668
CAS No.:61281-20-7
- Apigenin-7-diglucuronide
Catalog No.:BCX0667
CAS No.:74696-01-8
- Safranal
Catalog No.:BCX0666
CAS No.:116-26-7
- N-benzylpentadecanamide
Catalog No.:BCX0665
CAS No.:1572037-13-8
- Isotoosendanin
Catalog No.:BCX0664
CAS No.:97871-44-8
- 6''-O-apiosyl-Visammioside
Catalog No.:BCX0663
CAS No.:2254096-97-2
- Ophiopogonside A
Catalog No.:BCX0662
CAS No.:2423917-90-0
- Azorubin
Catalog No.:BCX0661
CAS No.:3567-69-9
- Filbertone
Catalog No.:BCX0660
CAS No.:81925-81-7
- 6'-O-galloylalbiflorin
Catalog No.:BCX0659
CAS No.:929042-36-4
- Sorbitol
Catalog No.:BCX0672
CAS No.:50-70-4
- Urolithin A
Catalog No.:BCX0673
CAS No.:1143-70-0
- 5,7-Dihydroxycoumarin
Catalog No.:BCX0674
CAS No.:2732-18-5
- Dendronobilin B
Catalog No.:BCX0675
CAS No.:1002717-96-5
- Polyporusterone A
Catalog No.:BCX0676
CAS No.:141360-88-5
- Cis-Emodin bianthrone
Catalog No.:BCX0677
CAS No.:61281-19-4
- Anhydrosafflor yellow B
Catalog No.:BCX0678
CAS No.:184840-84-4
- Emodin anthrone
Catalog No.:BCX0679
CAS No.:491-60-1
- Polyporusterone B
Catalog No.:BCX0680
CAS No.:141360-89-6
- 1-Methyl Emodin
Catalog No.:BCX0681
CAS No.:3775-08-4
- Maceneolignan H
Catalog No.:BCX0682
CAS No.:1314042-85-7
- 2-hydroxyl emodin-1-methyl ether
Catalog No.:BCX0683
CAS No.:346434-45-5
The protective effect of Ergolide in osteoarthritis: In vitro and in vivo studies.[Pubmed:38157693]
Int Immunopharmacol. 2024 Jan 25;127:111355.
Osteoarthritis (OA), a prevalent degenerative condition, occurs due to the deterioration of joint tissues and cells. Consequently, safeguarding chondrocytes against damage caused by inflammation is an area of future research emphasis. There is growing evidence that Ergolide (ERG) has multiple biological functions. Nevertheless, it is still uncertain whether it can hinder the advancement of OA. In this study, we investigate the ERG's potential to reduce inflammation and protect cartilage. ERG treatment in vitro effectively inhibited the excessive production of pro-inflammatory substances, such as inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX2), and tumor necrosis factor-alpha (TNF-alpha), leading to their complete suppression. Furthermore, ERG suppressed the production of matrix-degrading enzymes (ADAMTS-5) and matrix metalloproteinase 13 (MMP13), consequently impeding the breakdown of extracellular matrix (ECM) and restraining the synthesis of collagenase II and Aggrecan. Through the P38/MAPK pathway, we discovered that ERG hinders the activation of NF-kappaB in chondrocytes induced by IL-1beta. The protective effect of ERG was enhanced by the p38 MAPK inhibitor SB203580. In vivo, ERG further demonstrated protective effects on cartilage in animal models of DMM. In conclusion, the study has discovered that ERG exhibits innovative therapeutic potential in the context of OA.
Ergolide mediates anti-cancer effects on metastatic uveal melanoma cells and modulates their cellular and extracellular vesicle proteomes.[Pubmed:37981907]
Open Res Eur. 2023 Nov 13;3:88.
BACKGROUND: Uveal melanoma is a poor prognosis cancer. Ergolide, a sesquiterpene lactone isolated from Inula Brittanica, exerts anti-cancer properties. The objective of this study was to 1) evaluate whether Ergolide reduced metastatic uveal melanoma (MUM) cell survival/viability in vitro and in vivo; and 2) to understand the molecular mechanism of Ergolide action. METHODS: Ergolide bioactivity was screened via long-term proliferation assay in UM/MUM cells and in zebrafish MUM xenograft models. Mass spectrometry profiled proteins modulated by Ergolide within whole cell or extracellular vesicle (EVs) lysates of the OMM2.5 MUM cell line. Protein expression was analyzed by immunoblots and correlation analyses to UM patient survival used The Cancer Genome Atlas (TCGA) data. RESULTS: Ergolide treatment resulted in significant, dose-dependent reductions (48.5 to 99.9%; p<0.0001) in OMM2.5 cell survival in vitro and of normalized primary zebrafish xenograft fluorescence (56%; p<0.0001) in vivo, compared to vehicle controls. Proteome-profiling of Ergolide-treated OMM2.5 cells, identified 5023 proteins, with 52 and 55 proteins significantly altered at 4 and 24 hours, respectively ( p<0.05; fold-change >1.2). Immunoblotting of heme oxygenase 1 (HMOX1) and growth/differentiation factor 15 (GDF15) corroborated the proteomic data. Additional proteomics of EVs isolated from OMM2.5 cells treated with Ergolide, detected 2931 proteins. There was a large overlap with EV proteins annotated within the Vesiclepedia compendium. Within the differentially expressed proteins, the proteasomal pathway was primarily altered. Interestingly, BRCA2 and CDKN1A Interacting Protein (BCCIP) and Chitinase Domain Containing 1 (CHID1), were the only proteins significantly differentially expressed by Ergolide in both the OMM2.5 cellular and EV isolates and they displayed inverse differential expression in the cells versus the EVs. CONCLUSIONS: Ergolide is a novel, promising anti-proliferative agent for UM/MUM. Proteomic profiling of OMM2.5 cellular/EV lysates identified candidate pathways elucidating the action of Ergolide and putative biomarkers of UM, that require further examination.
Fermentation of Inula britannica using Lactobacillus plantarum SY12 increases of epigallocatechin gallate and attenuates toxicity.[Pubmed:37454617]
Food Chem. 2023 Dec 15;429:136844.
This study aimed to increase epigallocatechin gallate (EGCG) levels and attenuate the toxicity in Inulabritannica by fermentation using Lactobacillus plantarum SY12. The optimal medium was composed of 10 g of I. britannica, 4 g of xylose, 5 g of soytone, and 5 g of beef extract. The predicted value of EGCG was 237.327 mug/mL. To investigate damage in HepG2 cell lines by I. britannica extracts (IE) or fermented I. britannica extracts (FIE), cell viability, mitochondria membrane potential, the expression of apoptosis and autophagy genes, and chemical composition were measured. FIE increased cell viability, regulation of the gene expression (decreased p53, p62, p-ERK 1/2, and p-p38; increased CDK2 and CDK4) compared with IE. These results were explained by an increase in 1,3-dicaffeoylquinic acid and a decrease in 1-O-caffeoylquinic acid, 1-O-acetylbritannilactone, and Ergolide in FIE. In conclusion, these results indicated that fermentation can mitigate the toxicity in I. britannica.
Ergolide covalently binds NLRP3 and inhibits NLRP3 inflammasome-mediated pyroptosis.[Pubmed:37182452]
Int Immunopharmacol. 2023 Jul;120:110292.
BACKGROUND: NLR family pyrin domain-containing 3 (NLRP3)-mediated pyroptosis plays a key role in various acute and chronic inflammatory diseases. Targeted inhibition of NLRP3-mediated pyroptosis may be a potential therapeutic strategy for various inflammatory diseases. Ergolide (ERG) is a sesquiterpene lactone natural product derived from the traditional Chinese medicinal herb, Inula britannica. ERG has been shown to have anti-inflammatory and anti-cancer activities, but the target is remains unknown. HYPOTHESIS/PURPOSE: This study performed an in-depth investigation of the anti-inflammatory mechanism of ERG in NLRP3-mediated pyroptosis and NLPR3 inflammasome related sepsis and acute lung injury model. METHODS: ELISA and Western blot were used to determine the IL-1beta and P20 levels. Co-immunoprecipitation assays were used to detect the interaction between proteins. Drug affinity response target stability (DARTS) assays were used to explore the potential target of ERG. C57BL/6J mice were intraperitoneally injected with E. coli DH5alpha (2 x 10(9) CFU/mouse) to establish a sepsis model. Acute lung injury was induced by intratracheal administrationof lipopolysaccharide in wild-type mice and NLRP3 knockout mice with or without ERG treatment. RESULTS: We showed that ERG is an efficient inhibitor of NLRP3-mediated pyroptosis in the first and second signals of NLRP3 inflammasome activation. Furthermore, we demonstrated that ERG irreversibly bound to the NACHT domain of NLRP3 to prevent the assembly and activation of the NLRP3 inflammasome. ERG remarkably improved the survival rate of wild-type septic mice. In lipopolysaccharide-induced acute lung injury model, ERG alleviated acute lung injury of wild-type mice but not NLRP3 knockout mice. CONCLUSION: Our results revealed that the anti-pyroptosis effect of ERG are dependent on NLRP3 and NLRP3 NACHT domain is ERG's direct target. Therefore, ERG can serve as a precursor drug for the development of novel NLRP3 inhibitors to treat NLRP3 inflammasome mediated inflammatory diseases.
Inula britannica fermented with probiotic Weissella cibaria D30 exhibited anti-inflammatory effect and increased viability in RAW 264.7 cells.[Pubmed:32296568]
Food Sci Biotechnol. 2019 Oct 15;29(4):569-578.
The objective of this study was to increase the bioavailability of Inula britannica (IB) through fermentation with probiotic Weissella cibaria D30, and to evaluate the chemical composition, viability, and anti-inflammatory effect of fermented I. britannica (FIB). IB was fermented with W. cibaria D30 at 37 degrees C for 24 h. FIB increased total phenolic content and decreased total flavonoid content of IB. 1-O-acetylbritannilactone and Ergolide production, which are associated with the viability, increased from 1.38 to 4.13 mug/mg, and decreased from 5.24 to 0.94 mug/mg, in the control and FIB, respectively. In addition, the cell viability of RAW264.7 cells increased when pretreated with 400 mug/mL FIB. FIB inhibited the production of nitric oxide and proinflammatory cytokines by inhibiting NF-kappaB and MAPKs pathways. Therefore, FIB with W. cibaria D30 reduced the toxicity and increased the anti-inflammatory properties. These results indicate that FIB is a potential beneficial bioactive agent for functional foods.
Ergolide, a potent sesquiterpene lactone induces cell cycle arrest along with ROS-dependent apoptosis and potentiates vincristine cytotoxicity in ALL cell lines.[Pubmed:31904493]
J Ethnopharmacol. 2020 May 10;253:112504.
ETHNOPHARMACOLOGICAL RELEVANCE: Inula oculus christi belongs to the family of Asteraceae and it was traditionally wide used in treatment of kidney stones and urethra infection; besides, recently the potent sesquiterpene lactones isolated from inula species has gained increasing attention in cancer treatments. This study investigates the anti-cancer properties and underlying mechanism of Ergolide isolated from Inula oculus christi against leukemic cell lines. METHODS: Viability, metabolic activity and proliferation evaluated using different index of MTT assay such as IC(50) and GI(50). Human erythrocytes were used to evaluate hemolytic activity. Flow-cytometry was used to detect and measure ROS level, and the induction of apoptosis and autophagy were evaluated using Annexin V/PI, Acridine Orange staining, respectively. Moreover, qRT-PCR was performed to examine the expression of a large cohort of crucial regulatory genes. Tunel assay was also carried out to assess morphologically Ergolide effects. RESULTS: Ergolide did not exert ant cytotoxicity against non-tumorous cells and did not cause noticeable hemolysis. It also caused ROS production during early hours after treatment of cells which was then followed by cell cycle arrest in G0/G1 phase and autophagy induction. Using N-acetyl-L-cysteine (NAC), we found that Ergolide could not increase ROS and induce autophagy and moreover repressed cell death, indicating that Ergolide induce cell death through ROS-dependent manner by altering the expression of pro apoptotic related genes. Autophagy inhibition also potentiated Ergolide-induced cell death. Furthermore, Ergolide intensified vincristine cytotoxicity against acute lymphoblastic leukemia (ALL) cell lines revealed robust synergistic properties of Ergolide with VCR. CONCLUSION: Here we showed that Ergolide could be considered as a potent natural compound against leukemic cells by inducing cell cycle arrest followed by dose-dependent cell death. Based on results, Autophagy response in a result of ROS accumulation acted as a survival pathway and blocking this pathway could noticeably increase Ergolide cytotoxicity on ALL cell lines.
Sesquiterpene lactones from Inula helianthus-aquatica.[Pubmed:22993986]
Zhongguo Zhong Yao Za Zhi. 2012 Jun;37(11):1586-9.
OBJECTIVE: To investigate the sesquiterpene lactones of the aerial parts of Inula helianthus-aquatica. METHOD: Compounds were isolated and purified by silica gel, Sephadex LH-20 and preparative HPLC. On the basis of physicochemical properties and spectroscopic data, their structures were identified. RESULT: Seven sesquiterpene lactones and four other compounds were obtained and identified as 2-desoxy-4-epi-pulchellin (1), 6-acetoxy-4-hydroxy-1, 10H-pseudoguaia-11 (13)-en-12,8-olide (2), 4-acetoxy-6-hydroxy-1, 10H-pseudoguaia-11(13)-en-12,8-olide (3), 8-epi-inuviscolide (4), 2,3,11,13-tetrahydroaromaticin (5), 11,13-dihydro-Ergolide (6), 4-epipulchellin-2-O-acetate (7), 7-epiloliolide (8), loliolide (9), beta-sitosterol (10) and daucosterol (11). CONCLUSION: All the compounds were isolated from this plant for the first time.
[Simultaneous determination of three sesquiterpene lactones in Inula hupehensis by RP-HPLC].[Pubmed:22256758]
Zhongguo Zhong Yao Za Zhi. 2011 Sep;36(18):2520-4.
OBJECTIVE: A RP-HPLC method was developed for simultaneous determination of bigelovin, Ergolide and tomentosin in Inula hupehensis. METHOD: An Agilent C18 column (4.6 mm x 250 mm, 5 microm) was used for separation at 40 degrees C. The mobile phase was acetonitrile-water, and the flow rate was 1.2 mL x min(-1). The detection wavelength was set at 210 nm. RESULT: The method has good linearity in the ranges of 0.01792-0.1792 g x L(-1) (r =0.9999) for bigelovin, 0.0424-0.4240 g x L(-1) (r =0.9996) for Ergolide, and 0.044 8-0.4480 g x L(-1) (r = 0.9996) for tomentosin. The average recoveries of bigelovin, Ergolide, and tomentosin were 98.5%, 98.2%, 98.4%, with the RSD of 1. 3%, 1.3%, 1.7%, respectively. The results demonstrated that there was a significant difference in the contents of three sequterpene lactones among the tested Inulae Flos. CONCLUSION: The results indicated that the present RP-HPLC method is simple, quick and accurate, and can be used for the quality control of I. hupehensis, especially for the authentication of Inulae Flos.
Sesquiterpene lactones from Inula oculus-christi.[Pubmed:20433061]
Nat Prod Commun. 2010 Apr;5(4):511-4.
Inula oculus-christi L. (Compositae) extract was chromatographed and three sesquiterpene lactones Ergolide, gaillardin and pulchellin C were isolated. The structures of these compounds were determined by analysis of their spectroscopic data, and their crystal structures were defined using X-ray crystallography; the isolation of Ergolide and pulchellin C is reported for the first time from this species. These three compounds were evaluated for their in vitro cytotoxic activity against MDBK, MCF7 and WEHI164 cells; Ergolide and gaillardin exhibited lower and significantly different IC50 values compared with pulchellin C (p<0.001).
Suppression of the NF-kappaB signalling pathway by ergolide, sesquiterpene lactone, in HeLa cells.[Pubmed:17430640]
J Pharm Pharmacol. 2007 Apr;59(4):561-6.
We have previously reported that Ergolide, a sesquiterpene lactone isolated from Inula britannica, suppresses inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression by inhibiting nuclear factor-kappaB (NF-kappaB) in RAW 264.7 macrophages. In this study, we show that Ergolide suppresses the DNA binding activity of NF-kappaB and nuclear translocation of NF-kappaB p65 subunit, leading to the inhibition of NF-kappaB-dependent gene transcription in 12-O-tetradecanoylphorbol 13acetate (TPA)-stimulated HeLa cells. We also show that Ergolide decreases the degradation and phosphorylation of IkappaB, an inhibitory protein of NF-kappaB, and this effect is accompanied by a simultaneous reduction of IkappaB kinase (IKK) activity. However, Ergolide does not inhibit in-vitro IKK activity directly, suggesting the possible involvement of upstream IKK kinases in the regulation of NF-kappaB activation. Furthermore, Ergolide-mediated protein kinase Calpha (PKCalpha) inhibition is involved in reduction of NF-kappaB inhibition, as demonstrated by the observation that dominant negative PKCalpha, but not p44/42 MAPK and p38 MAPK, inhibits TPA-stimulated reporter gene expression. Taken together, our results suggest that Ergolide suppresses NF-kappaB activation through the inhibition of PKCalpha-IKK activity, providing insight for PKCalpha as a molecular target for anti-inflammatory drugs.
Apoptotic potential of sesquiterpene lactone ergolide through the inhibition of NF-kappaB signaling pathway.[Pubmed:16354403]
J Pharm Pharmacol. 2005 Dec;57(12):1591-7.
Treatment with Ergolide, a sesquiterpene lactone from Inula britannica var chinensis, caused the induction of apoptosis in Jurkat T cells, which was confirmed by DNA fragmentation, caspase-3 activation and cleavage of poly(ADP-ribose) polymerase in response to Ergolide. Furthermore, mitochondrial dysfunction appeared to be associated with Ergolide-induced apoptosis, because Bax translocation and cytochrome c release were stimulated by Ergolide. In parallel, the nuclear factor-kappaB (NF-kappaB) signaling pathway was significantly inhibited by Ergolide, which was accompanied by down-regulation of cell survival molecules, such as X-chromosome-linked inhibitor of apoptosis and Bcl-2. In addition, the JNK signaling pathway was involved in Ergolide-induced apoptosis. Collectively, our results identified a new mechanism for the anti-cancer property of Ergolide, attributable to the induction of apoptosis through down-regulation of cell survival signal molecules resulting from inhibition of the NF-kappaB signaling pathway.
Pseudoguaianolides isolated from Inula britannica var. chinenis as inhibitory constituents against inducible nitric oxide synthase.[Pubmed:12009027]
Arch Pharm Res. 2002 Apr;25(2):151-3.
Three pseudoguaianolide type sesquiterpenes, bigelovin (1), 2,3-dihydroaromaticin (2), and Ergolide (3) were isolated as inhibitory constituents against inducible nitric oxide synthase (iNOS) from the flowers of Inula britannica var. chinensis. Bigelovin (1) exhibited a highly potent inhibitory activity on lipopolysaccharide (LPS)-induced iNOS in murine macrophage RAW 264.7 cells with an IC50 value of 0.46 mM, which is about 8 times more potent than the known selective inhibitor of iNOS, L-N6-(1-iminoethyl)lysine (IC50 3.49 microM). 2,3-Dihydroaromaticin (2) and Ergolide (3) also exhibited potent inhibitory activities on LPS-induced iNOS with IC50 values of 1.05 and 0.69 microM, respectively.
Ergolide, sesquiterpene lactone from Inula britannica, inhibits inducible nitric oxide synthase and cyclo-oxygenase-2 expression in RAW 264.7 macrophages through the inactivation of NF-kappaB.[Pubmed:11399667]
Br J Pharmacol. 2001 Jun;133(4):503-12.
We investigated the mechanism of suppression of inducible nitric oxide synthase (iNOS) and cyclo-oxygenase-2 (COX-2) by Ergolide, sesquiterpene lactone from Inula britannica. iNOS activity in cell-free extract of LPS/IFN-gamma-stimulated RAW 264.7 macrophages was markedly attenuated by the treatment with Ergolide. Its inhibitory effect on iNOS was paralleled by decrease in nitrite accumulation in culture medium of LPS/IFN-gamma-stimulated RAW 264.7 macrophages in a concentration-dependent manner. However, its inhibitory effect does not result from direct inhibition of the catalytic activity of NOS. Ergolide markedly decreased the production of prostaglandin E(2) (PGE(2)) in cell-free extract of LPS/IFN-gamma-stimulated RAW 264.7 macrophages in a concentration-dependent manner, without alteration of the catalytic activity of COX-2 itself. Ergolide decreased the level of iNOS and COX-2 protein, and iNOS mRNA caused by stimulation of LPS/IFN-gamma in a concentration-dependent manner, as measured by Western blot and Northern blot analysis, respectively. Ergolide inhibited nuclear factor-kappaB (NF-kappaB) activation, a transcription factor necessary for iNOS and COX-2 expression in response to LPS/IFN-gamma. This effect was accompanied by the parallel reduction of nuclear translocation of subunit p65 of NF-kappaB as well as IkappaB-alpha degradation. In addition, these effects were completely blocked by treatment of cysteine, indicating that this inhibitory effect of Ergolide could be mediated by alkylation of NF-kappaB itself or an upstream molecule of NF-kappaB. Ergolide also directly inhibited the DNA-binding activity of active NF-kappaB in LPS/IFN-gamma-pretreated RAW 264.7 macrophages. These results demonstrate that the suppression of NF-kappaB activation by Ergolide might be attributed to the inhibition of nuclear translocation of NF-kappaB resulted from blockade of the degradation of IkappaB and the direct modification of active NF-kappaB, leading to the suppression of the expression of iNOS and COX-2, which play important roles in inflammatory signalling pathway.
Cytotoxic sesquiterpene lactones from Inula britannica.[Pubmed:9933993]
Planta Med. 1998 Dec;64(8):752-4.
Cytotoxicity-guided fractionation of the flowers of Inula britannica led to the isolation of four sesquiterpene lactones, 4 alpha, 6 alpha-dihydroxyeudesman-8 beta, 12-olide (1), Ergolide (2), 8-epi-helenalin (3), and bigelovin (4). Compound 1 was isolated as a new natural product. These compounds showed cytotoxicity against human tumor cell lines.
Cytotoxicity and NMR spectral assignments of ergolide and bigelovin.[Pubmed:8657753]
Planta Med. 1996 Apr;62(2):166-8.
Two potent cytotoxic sesquiterpene lactones, Ergolide (1) and bigelovin (2) were isolated from Inula hupehensis I. helianthus-aquatica and their structures and NMR data were assignment unambiguously by using a combination of one-and two-dimensional NMR techniques and computer modeling calculations.


