Polyporusterone BCAS# 141360-89-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 141360-89-6 | SDF | Download SDF |
| PubChem ID | 15168041.0 | Appearance | Powder |
| Formula | C28H44O6 | M.Wt | 476.65 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
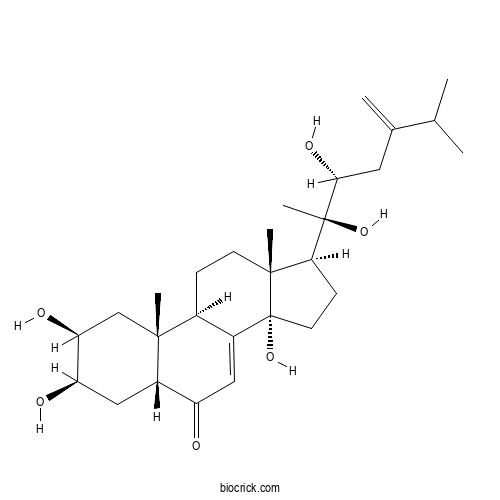
| Chemical Name | (2S,3R,5R,9R,10R,13R,14S,17S)-17-[(2R,3R)-2,3-dihydroxy-6-methyl-5-methylideneheptan-2-yl]-2,3,14-trihydroxy-10,13-dimethyl-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one | ||
| SMILES | CC(C)C(=C)CC(C(C)(C1CCC2(C1(CCC3C2=CC(=O)C4C3(CC(C(C4)O)O)C)C)O)O)O | ||
| Standard InChIKey | OQDKHYZVFZGSRC-DWJOQFFMSA-N | ||
| Standard InChI | InChI=1S/C28H44O6/c1-15(2)16(3)11-24(32)27(6,33)23-8-10-28(34)18-12-20(29)19-13-21(30)22(31)14-25(19,4)17(18)7-9-26(23,28)5/h12,15,17,19,21-24,30-34H,3,7-11,13-14H2,1-2,4-6H3/t17-,19-,21+,22-,23-,24+,25+,26+,27+,28+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Polyporusterone B Dilution Calculator

Polyporusterone B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.098 mL | 10.4899 mL | 20.9798 mL | 41.9595 mL | 52.4494 mL |
| 5 mM | 0.4196 mL | 2.098 mL | 4.196 mL | 8.3919 mL | 10.4899 mL |
| 10 mM | 0.2098 mL | 1.049 mL | 2.098 mL | 4.196 mL | 5.2449 mL |
| 50 mM | 0.042 mL | 0.2098 mL | 0.4196 mL | 0.8392 mL | 1.049 mL |
| 100 mM | 0.021 mL | 0.1049 mL | 0.2098 mL | 0.4196 mL | 0.5245 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Emodin anthrone
Catalog No.:BCX0679
CAS No.:491-60-1
- Anhydrosafflor yellow B
Catalog No.:BCX0678
CAS No.:184840-84-4
- Cis-Emodin bianthrone
Catalog No.:BCX0677
CAS No.:61281-19-4
- Polyporusterone A
Catalog No.:BCX0676
CAS No.:141360-88-5
- Dendronobilin B
Catalog No.:BCX0675
CAS No.:1002717-96-5
- 5,7-Dihydroxycoumarin
Catalog No.:BCX0674
CAS No.:2732-18-5
- Urolithin A
Catalog No.:BCX0673
CAS No.:1143-70-0
- Sorbitol
Catalog No.:BCX0672
CAS No.:50-70-4
- Ergolide
Catalog No.:BCX0671
CAS No.:54999-07-4
- Ankaflavin
Catalog No.:BCX0670
CAS No.:50980-32-0
- Bigelovin
Catalog No.:BCX0669
CAS No.:3668-14-2
- Trans-Emodin bianthrone
Catalog No.:BCX0668
CAS No.:61281-20-7
- 1-Methyl Emodin
Catalog No.:BCX0681
CAS No.:3775-08-4
- Maceneolignan H
Catalog No.:BCX0682
CAS No.:1314042-85-7
- 2-hydroxyl emodin-1-methyl ether
Catalog No.:BCX0683
CAS No.:346434-45-5
- Quinquenoside R1
Catalog No.:BCX0684
CAS No.:85013-02-1
- Sequoyitol
Catalog No.:BCX0685
CAS No.:523-92-2
- Cassiaside C2
Catalog No.:BCX0686
CAS No.:1958039-40-1
- 3-Hydroxy-1,2-dimethoxy-anthraquinone
Catalog No.:BCX0687
CAS No.:10383-62-7
- Gentianose
Catalog No.:BCX0688
CAS No.:25954-44-3
- Koumidine
Catalog No.:BCX0689
CAS No.:1358-75-4
- Farnesene
Catalog No.:BCX0690
CAS No.:502-61-4
- 8,9-epoxy-3,10-diisobutyryloxythymol
Catalog No.:BCX0691
CAS No.:22518-06-5
- N-(3-methoxybenzyl)-octadecanamide
Catalog No.:BCX0692
CAS No.:1429659-99-3
Rapid screening, isolation, and activity evaluation of potential xanthine oxidase inhibitors in Polyporus umbellatus and mechanism of action in the treatment of gout.[Pubmed:37798938]
Phytochem Anal. 2024 Jan;35(1):116-134.
INTRODUCTION: Studies show that Polyporus umbellatus has some pharmacological effects in enhancing immunity and against gout. OBJECTIVES: We aimed to establish new techniques for extraction, biological activity screening, and preparation of xanthine oxidase inhibitors (XODIs) from P. umbellatus. METHODS: First, the extraction of P. umbellatus was investigated using the back propagation (BP) neural network genetic algorithm mathematical regression model, and the extraction variables were optimised to maximise P. umbellatus yield. Second, XODIs were rapidly screened using ultrafiltration, and the change of XOD activity was tested by enzymatic reaction kinetics experiment to reflect the inhibitory effect of active compounds on XOD. Meanwhile, the potential anti-gout effects of the obtained active substances were verified using molecular docking, molecular dynamics simulations, and network pharmacology analysis. Finally, with activity screening as guide, a high-speed countercurrent chromatography (HSCCC) method combined with consecutive injection and two-phase solvent system preparation using the UNIFAC mathematical model was successfully developed for separation and purification of XODIs, and the XODIs were identified using MS and NMR. RESULTS: The results verified that polyporusterone A, Polyporusterone B, ergosta-4,6,8(14),22-tetraen-3-one, and ergosta-7,22-dien-3-one of P. umbellatus exhibited high biological affinity towards XOD. Their structures have been further identified by NMR, indicating that the method is effective and applicable for rapid screening and identification of XODIs. CONCLUSION: This study provides new ideas for the search for natural XODIs active ingredients, and the study provide valuable support for the further development of functional foods with potential therapeutic benefits.
Cytotoxic steroids from Polyporus umbellatus.[Pubmed:20458671]
Planta Med. 2010 Oct;76(15):1755-8.
The steroids ergone (1), (22E, 24R)-ergosta-7,22-dien-3beta-ol (2), 5alpha,8alpha-epidioxy-(22E,24R) -ergosta-6,22-dien-3beta-ol (3), ergosta-6,22-dien-3beta,5alpha,6beta-triol (4), and Polyporusterone B (5) were isolated from Polyporus umbellatus by bioassay-guided approach. They showed potent anticancer activity against HepG2 cells. Ergone displayed remarkable anticancer activity against HepG2, Hep-2, and Hela cancer cells, of which HepG2 cells were the most sensitive. Furthermore, the cytotoxic effects of ergone on normal human cells (HUVEC) were smaller than on cancer cells. The results showed that ergone had more selective cytotoxic activity against cancer cells than against normal cells.
Inhibitory effects of triterpenes isolated from Chuling (Polyporus umbellatus Fries) on free radical-induced lysis of red blood cells.[Pubmed:15863885]
Biol Pharm Bull. 2005 May;28(5):817-21.
Chuling, sclerotia of Polyporus umbellatus FRIES, has long been used for urological disorders in traditional medicine. In this study, we demonstrated that Chuling in vitro protects red blood cells from 2,2-azo-bis(2-amidinopropane)dihydrochloride (AAPH)-induced hemolysis. The inhibitory effect was dose-dependent at concentrations of 50 to 1000 microg/ml. Moreover, tests were carried out to identify the main ingredient of Chuling with scavenging effect on free radicals. Triterpene carboxylic acids isolated from the methanol extract of Chuling, namely, polyporusterone A and Polyporusterone B, were found to have inhibitory activities against AAPH-induced lysis of red blood cells. The anti-hemolytic effect was significantly stronger in Polyporusterone B compared with polyporusterone A. Furthermore, the ingestion of 150 mg of Chuling was associated with a significant increase in free-radical scavenging effect of plasma in rats.
Studies of the active substances in herbs used for hair treatment. II. Isolation of hair regrowth substances, acetosyringone and polyporusterone A and B, from Polyporus umbellatus Fries.[Pubmed:10598026]
Biol Pharm Bull. 1999 Nov;22(11):1189-92.
Fractionation of the 50% ethanol extract of Polyporus umbellatus Fries by column chromatography on Amberlite XAD-2, silica gel, Sephadex LH-20 and octadecyl silica gel (ODS) (C18)) monitored by a hair-regrowth activity assay, afforded three active principles, 1, 2 and 3. The structures of 1, 2 and 3 were determined as acetosyringone, polyporusterone A, and Polyporusterone B by comparison of their spectral data with that of authentic samples, respectively. The effects of several compounds related to acetosyringone, 3,4-dihydroxybenzaldehyde or polyporusterone A on hair regrowth were also investigated.


