SequoyitolCAS# 523-92-2 |
- D-Pinitol
Catalog No.:BCN5842
CAS No.:10284-63-6
- L-Quebrachitol
Catalog No.:BCN2727
CAS No.:642-38-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 523-92-2 | SDF | Download SDF |
| PubChem ID | 439990.0 | Appearance | Powder |
| Formula | C7H14O6 | M.Wt | 194.18 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 5-O-methyl-myo-inositol | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
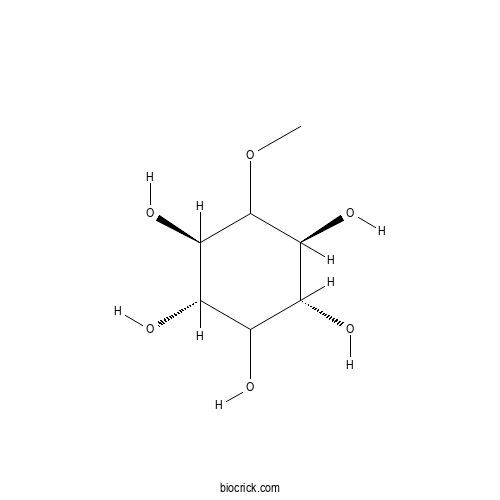
| Chemical Name | (1S,2R,4S,5R)-6-methoxycyclohexane-1,2,3,4,5-pentol | ||
| SMILES | COC1C(C(C(C(C1O)O)O)O)O | ||
| Standard InChIKey | DSCFFEYYQKSRSV-MVWKSXLKSA-N | ||
| Standard InChI | InChI=1S/C7H14O6/c1-13-7-5(11)3(9)2(8)4(10)6(7)12/h2-12H,1H3/t2?,3-,4+,5+,6-,7? | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Sequoyitol Dilution Calculator

Sequoyitol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.1499 mL | 25.7493 mL | 51.4986 mL | 102.9972 mL | 128.7465 mL |
| 5 mM | 1.03 mL | 5.1499 mL | 10.2997 mL | 20.5994 mL | 25.7493 mL |
| 10 mM | 0.515 mL | 2.5749 mL | 5.1499 mL | 10.2997 mL | 12.8747 mL |
| 50 mM | 0.103 mL | 0.515 mL | 1.03 mL | 2.0599 mL | 2.5749 mL |
| 100 mM | 0.0515 mL | 0.2575 mL | 0.515 mL | 1.03 mL | 1.2875 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Quinquenoside R1
Catalog No.:BCX0684
CAS No.:85013-02-1
- 2-hydroxyl emodin-1-methyl ether
Catalog No.:BCX0683
CAS No.:346434-45-5
- Maceneolignan H
Catalog No.:BCX0682
CAS No.:1314042-85-7
- 1-Methyl Emodin
Catalog No.:BCX0681
CAS No.:3775-08-4
- Polyporusterone B
Catalog No.:BCX0680
CAS No.:141360-89-6
- Emodin anthrone
Catalog No.:BCX0679
CAS No.:491-60-1
- Anhydrosafflor yellow B
Catalog No.:BCX0678
CAS No.:184840-84-4
- Cis-Emodin bianthrone
Catalog No.:BCX0677
CAS No.:61281-19-4
- Polyporusterone A
Catalog No.:BCX0676
CAS No.:141360-88-5
- Dendronobilin B
Catalog No.:BCX0675
CAS No.:1002717-96-5
- 5,7-Dihydroxycoumarin
Catalog No.:BCX0674
CAS No.:2732-18-5
- Urolithin A
Catalog No.:BCX0673
CAS No.:1143-70-0
- Cassiaside C2
Catalog No.:BCX0686
CAS No.:1958039-40-1
- 3-Hydroxy-1,2-dimethoxy-anthraquinone
Catalog No.:BCX0687
CAS No.:10383-62-7
- Gentianose
Catalog No.:BCX0688
CAS No.:25954-44-3
- Koumidine
Catalog No.:BCX0689
CAS No.:1358-75-4
- Farnesene
Catalog No.:BCX0690
CAS No.:502-61-4
- 8,9-epoxy-3,10-diisobutyryloxythymol
Catalog No.:BCX0691
CAS No.:22518-06-5
- N-(3-methoxybenzyl)-octadecanamide
Catalog No.:BCX0692
CAS No.:1429659-99-3
- N-benzyl-heptadecanamide
Catalog No.:BCX0693
CAS No.:883715-19-3
- Polygalasaponin XXVIII
Catalog No.:BCX0694
CAS No.:176182-01-7
- Cavidine
Catalog No.:BCX0695
CAS No.:32728-75-9
- Quercetin 3-O-[beta-D-xylosyl-(1->2)-beta-D-glucoside]
Catalog No.:BCX0696
CAS No.:83048-35-5
- Desacylsenegasaponin B
Catalog No.:BCX0697
CAS No.:163589-51-3
Cytotoxicity of seputhecarpan D, thonningiol and 12 other phytochemicals from African flora towards human carcinoma cells.[Pubmed:29378558]
BMC Complement Altern Med. 2018 Jan 30;18(1):36.
BACKGROUND: Despite the remarkable progress in cancer therapy in recent years, this disease still remains a serious public health concern. The use of natural products has been and continues to be one of the most effective ways to fight malignancies. The cytotoxicity of 14 compounds from African medicinal plants was evaluated in four human carcinoma cell lines and normal fibroblasts. The tested samples included: beta-spinasterol (1), friedelanone (2), 16beta-hydroxylupeol (3), beta-amyrin acetate (4), lupeol acetate (5), Sequoyitol (6), rhamnitrin (7), europetin 3-O-rhamnoside (8), thonningiol (9), glyasperin F (10), seputhecarpan B (11), seputhecarpan C (12), seputhecarpan D (13) and rheediaxanthone A (14). METHODS: The neutral red uptake (NR) assay was used to evaluate the cytotoxicity of samples; caspase-Glo assay, flow cytometry for cell cycle analysis and mitochondrial membrane potential (MMP) as well as spectrophotometry to measure levels of reactive oxygen species (ROS) were performed to detect the mode of action of compounds 9 and 13 in MCF-7 breast adenocarcinoma cells. RESULTS: Compounds 3, 9-13 displayed cytotoxic effects against the four tested cancer cell lines with IC(50) values below 85 muM. Compounds 9 and 13 had IC(50) values below 10 muM in 4/4 and 3/4 tested cell lines respectively. The IC(50) values varied from 0.36 muM (against MCF7 cells) to 5.65 muM (towards colon carcinoma DLD-1 cells) for 9, from 9.78 muM (against MCF7 cells) to 67.68 muM (against HepG2 cells) for 13 and 0.18 muM (towards HepG2 cells) to 72 muM (towards Caco-2 cells) for the reference drug, doxorubicin. Compounds 9 and 13 induced cell cycle arrest in Go/G1 whilst doxorubicin induced arrest in G2/M. The two molecules (9 and 13) also induced apoptosis in MCF-7 cells through activation of caspases 3/7 and 9 as well as enhanced ROS production. CONCLUSION: Compounds 9 and 13 are good cytotoxic phytochemicals that should be explored more in future to develop a cytotoxic drug to fight human carcinoma.
Two new pterocarpans and a new pyrone derivative with cytotoxic activities from Ptycholobium contortum (N.E.Br.) Brummitt (Leguminosae): revised NMR assignment of mundulea lactone.[Pubmed:28316643]
Chem Cent J. 2016 Oct 5;10:58.
BACKGROUND: Ptycholobium is a genus related to Tephrosia which comprises only three species. Compared to Tephrosia, which has been phytochemically and pharmacologically studied, Ptycholobium species have only few or no reports on their chemical constituents. Moreover, no studies on the cytotoxic activities of its secondary metabolites have been previously documented. RESULTS: From the non polar fractions of the roots bark of Ptycholobium contortum (syn Tephrosia contorta), two new pterocarpans: seputhecarpan C 1 and seputhecarpan D 2 and a new pyrone derivative, ptycholopyrone A 3 were isolated. Alongside, five known compounds identified as 3-alpha,alpha-dimethylallyl-4-methoxy-6-styryl-alpha-pyrone or mundulea lactone 4, glyasperin F 5, seputhecarpan A 6, seputheisoflavone 7 and 5-O-methyl-myo-inositol or Sequoyitol 8 were also obtained. Their structures were established by the mean means of spectroscopic data in conjunction to those reported in literature. The NMR assignment of the major compound mundulea lactone 4 is revised in this paper. In addition, the cytotoxicity of the isolated metabolites was evaluated on two lung cancer cell lines A549 and SPC212. 8 was not active while compounds 1, 2, 4-7 displayed antiproliferative effects against the two carcinoma cell lines with IC(50) values below 75 microM. IC(50) values below 10 microM were obtained for 4, 6 and 7 on SPC212 cells. CONCLUSION: Based on the obtained results, Ptycholobium contortum turns to be a rich source of phenolic metabolites among them some bearing prenyl moieties. This study reports for the first time the isolation of pyrone derivatives 3 and 4 from Ptycholobium genus. The cytotoxicity observed for the isolate is also reported for the first time and shows that 4, 6 and 7 could be chemically explored in order to develop a hit candidate against lung cancer. Graphical abstractTwo new pterocarpans and a new pyrone derivative with cytotoxic activities from ptycholobium contortum (N.E.Br.) Brummitt (Leguminosae): revised NMR assignment of mundulea lactone.
Content of methylated inositols in familiar edible plants.[Pubmed:25734537]
J Agric Food Chem. 2015 Mar 18;63(10):2683-8.
Familiar plants contain large amounts of inositols; soybean, white clover, red clover, bush clover, locust tree, wisteria, and kudzu of the legume family contain pinitol (3-O-methyl-chiro-inositol) at approximately 200-600 mg/100 g fresh weight (FW). The contents of pinitol in other plants were 260 mg/100 g FW for sticky mouse-ear, 275 mg/100 g FW for chickweed, and 332 mg/100 g FW for ginkgo. chiro-Inositol of 191 and 156 mg/100 g FW was also found in dandelion and Japanese mallotus, respectively. Ononitol (4-O-methyl-myo-inositol) of 166 mg/100 g FW was found in sticky mouse-ear. Furthermore, young leaves of ginkgo contained Sequoyitol (5-O-methyl-myo-inositol) of 287 mg/100 g FW. Hydroxyl radical scavenging activities of the methylated inositols were higher than those of the original inositols. Effective uses of these familiar edible plants are expected to promote good health.
[Effect of sequoyitol on expression of NOX4 and eNOS induced with glucose in human umbilical vein endothelial cells].[Pubmed:25016868]
Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2014 Mar;30(2):147-52.
OBJECTIVE: To investigate the protective effect and mechanism of Sequoyitol (Sep) on high glucose-induced human umbilical vein endothelial cells (HUVECs) injury. METHODS: HUVECs were cultured with high glucose (30 mmol/L) in the presence or absence of Sequoyitol (0.1, 1 and 10 micromol/L) for 24 h. Cell proliferation was measured by BrdU marking and cell cycle was detected by flow cytometry. 2', 7'-dichlorofluorescein diacetate was used to evaluate intracellular reactive oxygen species (ROS) levels. The NO, malonydialdehyde (MDA) and H2O2 levels were determined by colorimetric method according to the manufacturer's instructions. The expression of endothelial nitric oxide synthase (eNOS) and NADPH oxidase 4 (NOX4) were measured by real-time PCR and Western blot. RESULTS: In the present study, we found that Sequoyitol pretreatment for 1 h significantly decreased cell injury, promoted cell proliferation. Meanwhile Sequoyitol significantly down-regulated NOX4 expression and decreased the level of ROS, MDA and H2O2 and obviously increased NO levels and up-regulated eNOS expression. CONCLUSION: Sequoyitol alleviates high glucose-induced cell injuries in HUVECs via inhibiting oxidative stress and up-regulating eNOS expression.
[Effect of sequoyitol on expression of NOX4 and eNOS in aortas of type 2 diabetic rats].[Pubmed:24961103]
Yao Xue Xue Bao. 2014 Mar;49(3):329-36.
The aim of the present study is to investigate the effects of Sequoyitol (Seq) on expression of eNOS and NOX4 in aortas of type 2 diabetic rats. Type 2 diabetic rats induced by high fat and high sugar diet and low dose of streptozotocin (STZ, 35 mg x kg(-1)) and were administered Seq (12.5, 25 and 50 mg x kg(-1) x d(-1)) for 6 weeks. The fasting blood glucose (FBG) and body weight were tested. Acetylcholine (Ach) induced endothelium-dependent relaxation and sodium nitroprusside (SNP) induced endothelium-independent relaxation were measured in aortas for estimating endothelial function. Aortic morphological change was observed with HE staining. The level of serum insulin was measured by radioimmunoassay. The total antioxidative capacity (T-AOC), malondialdehyde (MDA) and NO levels in aortas were determined according to the manufacturer's instructions. In addition, the expressions of eNOS and NOX4 in aortas were measured by immunohistochemisty, real-time PCR or Western blotting. The results showed that Seq significantly decreased FBG and insulin resistance, and improved aortic endothelium-dependent vasorelaxation function. The expressions of NOX4 and MDA content were obviously decreased, while the expression of eNOS, the levels of NO and T-AOC increased significantly in aortas of diabetic rats with Seq treatment. In conclusion, Seq protects against aortic endothelial dysfunction of type 2 diabetic rats through down-regulating expression of NOX4 and up-regulating eNOS expression.
Sequoyitol ameliorates diabetic nephropathy in diabetic rats induced with a high-fat diet and a low dose of streptozotocin.[Pubmed:24784471]
Can J Physiol Pharmacol. 2014 May;92(5):405-17.
Sequoyitol decreases blood glucose, improves glucose intolerance, and enhances insulin signaling in ob/ob mice. The aim of this study was to investigate the effects of Sequoyitol on diabetic nephropathy in rats with type 2 diabetes mellitus and the mechanism of action. Diabetic rats, induced with a high-fat diet and a low dose of streptozotocin, and were administered Sequoyitol (12.5, 25.0, and 50.0 mg.(kg body mass)(-1).d(-1)) for 6 weeks. The levels of fasting blood glucose (FBG), serum insulin, blood urea nitrogen (BUN), and serum creatinine (SCr) were measured. The expression levels of p22(phox), p47(phox), NF-kappaB, and TGF-beta1 were measured using immunohistochemisty, real-time PCR, and (or) Western blot. The total antioxidative capacity (T-AOC), as well as the levels of malondialdehyde (MDA) and reactive oxygen species (ROS) were also determined. The results showed that Sequoyitol significantly decreased FBG, BUN, and SCr levels, and increased the insulin levels in diabetic rats. The level of T-AOC was significantly increased, while ROS and MDA levels and the expression of p22(phox), p47(phox), NF-kappaB, and TGF-beta1 were decreased with Sequoyitol treatment both in vivo and in vitro. These results suggested that Sequoyitol ameliorates the progression of diabetic nephropathy in rats, as induced by a high-fat diet and a low dose of streptozotocin, through its glucose-lowering effects, antioxidant activity, and regulation of TGF-beta1 expression.
Potent microbial and tyrosinase inhibitors from stem bark of Bauhinia rufescens (Fabaceae).[Pubmed:24354195]
Nat Prod Commun. 2013 Oct;8(10):1435-7.
The stem bark extracts of Bauhinia rufescens Lam. (Fabaceae) yielded 6-methoxy-7-methyl-8-hydroxydibenz[b,f]oxepin, alpha-amyrin acetate, beta-sitosterol 3-O-beta-D-xylopyranoside, 4-(2'-Hydroxyphenethyl)-5-methoxy-2-methylphenol, menisdaurin and Sequoyitol. Their structures were determined using spectroscopic methods and comparisons with the literature data. For the antimicrobial assay Gram-positive and Gram-negative bacterial and fungal strains were tested, while the tyrosinase inhibition assay utilized L-DOPA as a substrate for the tyrosinase enzyme. 6-Methoxy-7-methyl-8-hydroxydibenz[b,f]oxepin, a-amyrin acetate, beta-sitosterol 3-O-D-xylopyranoside, menisdaurin and Sequoyitol showed weak to moderate activities with minimum inhibition concentration (MIC) values in the range of 112.5-900 microg/mL against all bacterial strains, while the MIC values for the fungal strains were in the range of 28.1-450 microg/mL. In the tyrosinase inhibition assay, a-amyrin acetate was found to be moderately active against tyrosinase with an inhibition of 62% at 0.1 mg/mL. This activity was lower than that of the positive control, kojic acid (85%).
[Effects of sequoyitol on expression of NADPH oxidase subunits p22 phox and p47 phox in rats with type 2 diabetic liver disease].[Pubmed:23833934]
Yao Xue Xue Bao. 2013 Apr;48(4):489-94.
This study is to observe the effects of Sequoyitol on the expression of NADPH oxidase subunits p22 phox and p47 phox in rats with type 2 diabetic liver diseases. The model of high fat and high sugar diet as well as intraperitoneal injection of small dose of streptozotocin (STZ, 35 mg x kg(-1)) induced diabetic rat liver disease was used. After Sequoyitol (50, 25 and 12.5 mg x kg(-1)) was administrated for 6 weeks, the contents of blood glucose (BG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total antioxidant capacity (T-AOC), hydrogen peroxide (H2O2), NO and insulin (Ins) were measured, liver p22 phox and p47 phox mRNA content was determined with real-time PCR and the expression of p22 phox and p47 phox protein was examined by Western blotting. In addition, pathological changes in liver were observed with HE staining. Sequoyitol could reduce the content of fasting blood glucose, ALT, AST, Ins and H2O2, restore insulin sensitive index (ISI) and weight, elevate liver tissue T-AOC and NO content, reduce the NADPH oxidase subunit liver tissue p22 phox and p47 phox mRNA and protein expression, as well as ameliorate liver pathologic lesions. The results showed that Sequoyitol can ease the type 2 diabetic rat liver oxidative stress by lowering NADPH oxidase expression.
Herbal constituent sequoyitol improves hyperglycemia and glucose intolerance by targeting hepatocytes, adipocytes, and beta-cells.[Pubmed:22297305]
Am J Physiol Endocrinol Metab. 2012 Apr 15;302(8):E932-40.
The prevalence of insulin resistance and type 2 diabetes increases rapidly; however, treatments are limited. Various herbal extracts have been reported to reduce blood glucose in animals with either genetic or dietary type 2 diabetes; however, plant extracts are extremely complex, and leading compounds remain largely unknown. Here we show that 5-O-methyl-myo-inositol (also called Sequoyitol), a herbal constituent, exerts antidiabetic effects in mice. Sequoyitol was chronically administrated into ob/ob mice either orally or subcutaneously. Both oral and subcutaneous administrations of Sequoyitol decreased blood glucose, improved glucose intolerance, and enhanced insulin signaling in ob/ob mice. Sequoyitol directly enhanced insulin signaling, including phosphorylation of insulin receptor substrate-1 and Akt, in both HepG2 cells (derived from human hepatocytes) and 3T3-L1 adipocytes. In agreement, Sequoyitol increased the ability of insulin to suppress glucose production in primary hepatocytes and to stimulate glucose uptake into primary adipocytes. Furthermore, Sequoyitol improved insulin signaling in INS-1 cells (a rat beta-cell line) and protected INS-1 cells from streptozotocin- or H(2)O(2)-induced injury. In mice with streptozotocin-induced beta-cell deficiency, Sequoyitol treatments increased plasma insulin levels and decreased hyperglycemia and glucose intolerance. These results indicate that Sequoyitol, a natural, water-soluble small molecule, ameliorates hyperglycemia and glucose intolerance by increasing both insulin sensitivity and insulin secretion. Sequoyitol appears to directly target hepatocytes, adipocytes, and beta-cells. Therefore, Sequoyitol may serve as a new oral diabetes medication.
An oviposition stimulant binding protein in a butterfly: Immunohistochemical localization and electrophysiological responses to plant compounds.[Pubmed:19721890]
Commun Integr Biol. 2009 Jul;2(4):356-8.
Oviposition is evoked by plant compounds, which are recognized by chemoreceptive organs of insects. The swallowtail butterfly, Atrophaneura alcinous, oviposits its eggs on the host plant, Aristolochia debilis, in the presence of only two stimulating compounds: an alkaloid, aristolochic acid, and a monosaccharide, Sequoyitol. In our previous study, a unique protein of 23 kDa [Oviposition stimulant(s) binding protein (OSBP)] was found in the forelegs of female, but not male A. alcinous. The electrophysiological response of A. alcinous to an extract of A. debilis was depressed by the presence of OSBP antiserum, suggesting that OSBP presumably binds to oviposition stimulant(s). We show here, using a highly sensitive fluorescence micro-binding assay that native OSBP binds to a main oviposition stimulant, aristolochic acid, from its host plant, A. debilis. Three-dimensional molecular modeling studies also gave a reasonable structure for the OSBP/aristolochic acid complex. This is the first report of a native chemoreceptive protein binding to an oviposition stimulant ligand in insects.
Chemical constituents from Amentotaxus yunnanensis and Torreyayunnanensis.[Pubmed:12880325]
J Nat Prod. 2003 Jul;66(7):1002-5.
In a chemical study of taxonomically related Taxaceae plants of Yunnan Province, China, seven compounds, including a new amentoflavone biflavonoid, 2,3-dihydro-7,7' '-dimethoxyamentoflavone (1), were isolated from Amentotaxus yunnanensis, and 12 isolates were obtained from Torreya yunnanensis. From the latter plant, a new abietane diterpene, torreyayunnin (7), is reported for the first time. The known isolates from A. yunnanensis have been identified as sequoiaflavone (3), sotetsuflavone (4), 7,7' '-dimethoxyamentoflavone (5), lutein, beta-sitosterol, and Sequoyitol. Amentoflavone (2), sotetsuflavone (4), sciadopitysin (6), 12-hydroxydehydroabietinol, meridinol, balanophonin, (+)-pinoresinol monomethyl ether, (+)-pinoresinol monomethyl ether glucoside, erythro-1-(4-hydroxy-3-methoxyphenyl)-2-[4-[2-formyl-(E)-vinyl]-2- methoxyphenoxy]propane-1,3-diol, threo-1-(4-hydroxy-3-methoxyphenyl)-2- [4-[2-formyl-(E)-vinyl]-2-methoxyphenoxy] propane-1,3-diol, and (E)-2-butenedioic acid were identified as known isolates from T. yunnanensis. The presence of the amentoflavone biflavonoids (1, 3-5) in A. yunnanensis supports its placement in the Taxaceae. The occurrence of the biflavonoid sotetsuflavone (4) in both A. yunnanensis and T. yunnanensis suggests that these two genera are closely related. The identification and structural elucidation of these isolates were based on spectral data analysis including 1D and 2D NMR.


